Afr. J. Parasitol. Mycol. Entomol. 2023, 1(1), 4; doi:10.35995/ajpme1010004
Availability of Systemic Antifungals in Africa: A Comparative Study between North Africa and West Africa
1
Service of Parasitology-Mycology, Faculty of Medicine, Pharmacy and Odontology, Cheikh Anta Diop University, BO 3005, Dakar, Senegal
2
Laboratory of Parasitology and Mycology, Aristide Le Dantec University Hospital, BO 3001, Dakar, Senegal
3
Centre International de Recherche et de Formation en Génomique Appliquée, et de Surveillance Sanitaire (CIGASS), Cheikh Anta Diop University of Dakar, BO 16477, Dakar, Senegal
4
Department of Parasitology-Mycology-Animal Biology-Zoology, UFR Sciences pharmaceutiques et biologiques, Felix Houphouët-Boigny University, BO V34, Abidjan, Côte d’Ivoire
5
Laboratory of Parasitology-Mycology, National institute of Public Health, BO V34, Abidjan, Côte d’Ivoire
6
Laboratory of the « Mère et Enfant » Hospital Center, BO 40028, Nouakchott, Mauritania
7
UFR 2S, Assane Seck University of Ziguinchor, BO 523 Ziguinchor, Senegal
*
Corresponding author: khadimase@gmail.com; Phone: +221 77 437 04 61
How to cite: Diongue, K.; Miezan, A.J-S.; Sy, O.; Diop, A.; Diallo, M.A.; Seck, M.C.; Ndiaye, M.; Badiane, A.S.; Ndiaye, D. Availability of Systemic Antifungals in Africa: A Comparative Study between North Africa and West Africa. Afr. J. Parasitol. Mycol. Entomol., 2023, 1(1): 4; doi:10.35995/ajpme1010004.
Received: 31 December 2022 / Accepted: 15 May 2023 / Published: 13 June 2023
Abstract
:Introduction. Due to the lack of systemic antifungals (SAs), many invasive fungal infections (IFIs) do not yet benefit from effective treatment in Africa. Yet, fungi are a major threat to human health. This study aimed to assess the availability of SAs and compare this availability between West Africa (WA) and North Africa (NA). Materials and methods. The information processed was obtained during the same period between February and June 2021 in Senegal, Mauritania, and Côte d’Ivoire as well as in Morocco and Tunisia. In WA, data were received from wholesale drug suppliers and private pharmacies while in NA, the data were those available on the websites of the pharmacy and drug directorates. Results. All classes of SAs were available. However, this availability was not uniform depending on location, whether in WA or NA. Fluconazole and itraconazole were the only SA available in WA. In NA, all classes of SAs have been found, particularly in Morocco, while flucytosine was the only molecule absent in Tunisia. Conclusion. NA is ahead of WA as regards the burden of IFIs both diagnostically and in the availability of SAs. Studies on the epidemiology of IFIs in Africa, particularly in WA, are limited. The partial data available highlight that IFIs are not negligible, though these may be underestimated. Thus, a network based on the directory of African pharmacy and drug directorates must be set up and run in collaboration with the national laboratory directorates for the harmonization of procedures in order to reduce morbidity and mortality related to IFIs.
Keywords:
Systemic Antifungals; West Africa; North Africa; Fungal Infections1. Introduction
While the current antifungal arsenal in Africa has products that permit the treatment of superficial fungal infections, many invasive fungal infections (IFIs) do not yet benefit from effective treatment due to the lack of systemic antifungal (SAs) molecules [1]. However, fungi are ubiquitous; therefore, they are a major threat to human health, causing more than a billion cutaneous infections, more than 100 million mucous infections, 10 million serious allergies, and more than a million deaths each year [2]. Regrettably, these “fungal threats”, which are underestimated and neglected endanger millions of lives each year, and result in an estimated 1.7 million deaths per year worldwide [3]. This means that the overall mortality due to IFIs is higher than that due to parasites such as Plasmodium. This latter agent is responsible for malaria, which remains the leading parasitic endemic in the world causing 409,000 deaths in 2019 with 95% of these occurring in Sub-Saharan Africa [4]. This mortality is also higher than that of breast cancer (684,996 in 2020) [5], and that caused by Human Immunodeficiency Virus (HIV: 680,000 [480,000–1,000,000] in 2020) [6]. It is equivalent to the death rate from tuberculosis (bacterial infection) which is reported to cause 1.5 million deaths every year [2,7]. In addition, the number of cases continues to increase [3].
Even if the outbreak of the coronavirus disease 19 (COVID-19) has disrupted this trend causing 3,507,477 deaths globally and 86,611 in Africa [8] by May 29, 2021, the incidence of fungal infections continues to make headlines. Therefore, the availability of SAs should be a priority, particularly in Africa, to avoid reliving the same unforeseen circumstances recently experienced with the occurrence of the COVID-19. Indeed, the number of qualified human resources, and the availability of drugs and biomedical materials are challenges in this continent.
Antifungals—molecules used to treat fungal infections—fall into two categories. On the one hand, local antifungals or topicals are not absorbed orally—they are used for a local effect. On the other hand, SAs are absorbed orally or administered intravenously, as they diffuse in the viscera [9]. The latter group is divided into four classes: (1) polyenes with amphotericin B (AMB), (2) triazoles with fluconazole (FLC), itraconazole (ITC), voriconazole (VRC), posaconazole and isavuconazole, (3) echinocandins with caspofungin (CPF), anidulafungin (ANF), and micafungin (MCF) and (4) nucleoside analogs such as 5-fluorocytosine (flucytosine or 5-FC) [10,11]. For pharmacokinetic reasons, molecules such as griseofulvin (naturally occurring extract from Penicillium griseofulvum) or terbinafine (allylamines) are absorbed orally but are concentrated exclusively in the superficial skin layers. Thus, they are compared to topicals concerning therapeutic indications [9].
Given that we have already demonstrated the unavailability of SAs in Senegal in a previous article [1], this study aimed to assess the availability of SAs in other African countries, as well as to compare this availability between West Africa (WA) and North Africa (NA).
2. Material and Methods
2.1. Study Site and Period
We designed a cross-sectional survey on the availability of SA drugs. The survey was open between February and June 2021, and sent out to the “Réseau Médicaments et Développement (REMED)” via their professional directory (https://remed.org/wp-content/uploads/2016/07/Annuaire-Directions-de-la-pharma-Afrique_2015.pdf) which included 27 African countries and Haiti.
2.2. Study Design
First, 22 African directors were directly contacted by email. These directors were based in the following locations: one country in North Africa (Tunisia), three in the East (Comoros, Madagascar and Rwanda), eight in the Central Africa (Burundi, Cameroon, Central African Republic, Chad, Congo, Democratic Republic of Congo, Equatorial Guinea and Gabon), and ten in the West (Benin, Burkina Faso, Cabo Verde, Côte d’Ivoire, Guinea, Mali, Mauritania, Niger, Senegal, and Togo). Reminders were sent to the directors in cases of non-response.
The countries included were those with email addresses directly available from the REMED professional directory. Countries without an available email address among those listed on the REMED professional directory were not included.
Secondarily, faced with the lack of responses from the latter, in WA, data received from wholesale drug suppliers combined with data from the use of the national list of essential drugs in Senegal [12] and Mauritania [13] were exploited. Alternatively, data from the use of the national list of essential drugs in Côte d’Ivoire [14] combined with those received from seven (07) pharmacies including four in Abidjan (the capital) and three in other localities (Bouaké in the center, Korhogo in the north, and Man in the west, respectively 350, 635 and 509 km from Abidjan) were exploited in the face of the lack of responses from Ivorian wholesalers. Likewise, for NA, the data used were those available on the sites of the pharmacy and drug directorates of Morocco (http://dmp.sante.gov.ma/) and Tunisia (http://www.dpm.tn/) which were the only ones with antifungal data available online.
Finally, the information processed was obtained during the same period as specified above, in Senegal, Mauritania, and Côte d’Ivoire for WA, as well as in Morocco and Tunisia for NA (Figure 1).
2.3. Statistical Analysis
Data were collected and analysed with MS Excel 2016 and the results were presented as tables.
3. Results
Depending on the class of antifungals, all four classes of SAs (polyenes, azoles, echinocandins, and 5-fluorocytosine) were available in Africa. However, this availability was not uniform depending on location whether in WA or NA. Indeed, fluconazole and itraconazole—available in Côte d’Ivoire—were the only SAs available in WA: Côte d’Ivoire, Mauritania, and Senegal (Table 2).
On the other hand, in NA, all classes of antifungals have been found, particularly in Morocco, while flucytosine was the only molecule absent in Tunisia.
AMB, the only systemic polyene, was available in its desoxycholate form as an oral suspension and powder for injection in Morocco, while in Tunisia the latter galenic form was the only one available (Table 3).
For azoles, FLC and VRC were the only triazoles available in Morocco while in Tunisia, in addition to these two molecules, ITC was available. The galenic forms and the dosages of these molecules are detailed in Table 3.
Regarding echinocandins, only MCF was available in Morocco, while CPF and ANF were the only ones available in Tunisia. MCF is presented as a powder for IV infusion, whereas CPF and ANF are presented as powders for injection (Table 3).
4. Discussion
Periodically, the World Health Organization (WHO) publishes a list of essential drugs that it estimates to consist of minimum medical needs for a basic health care system. Thus, the WHO lists the most effective, safe, and cost-effective drugs for priority diseases with priority conditions selected based on estimated current and future relevance to public health, as well as treatment potential. The 2019 version of this list included the following as antifungals: AMB (50 mg powder for injection in its desoxycholate or liposomal form), 5-FC (2.5 g/250 mL by IV or 250 mg tablet), FLC (50 mg tablet or 50 mg/5 mL drinkable suspension or 2 mg/mL IV injection), ITC (100 mg tablet or 100 mg/mL drinkable suspension) and VRC (50 mg and 200 mg tablet or 40 mg/mL drinkable suspension or 200 mg powder for injection) [16].
Unfortunately, in Africa, particularly in Sub-Saharan Africa, the availability of these basic medicines remains a public health problem despite the regular updating of country-specific lists. This is the case with Senegal [12], and Mauritania [13]. Even though ITC is now available in Côte d’Ivoire [14]. Similarly, ITC would be available in Sudan (not included in this study) in particular at the Mycetoma Research Center (MRC) where it is used in the treatment of fungal mycetoma. Yet, Africa will be no exception to the emergence of global fungal infections. Indeed, medical advances have improved the quality of life of patients suffering from previously incurable diseases at the cost of deep and prolonged immunosuppression. These prolonged managements during which patients are submitted to prolonged treatment, receive broad-spectrum antibiotic therapy, and are subject to numerous invasive procedures such as central venous catheter may promote direct vascular access of Candida spp. following previous colonization [17].
One of the most prominent examples of this fungal opportunism leading to invasive and highly fatal fungal infections has been and still is the increasing incidence of cryptococcal meningitis with the advent of HIV. Sub-Saharan Africa remains the region hardest hit by HIV in the world: 67.7% of all people living with HIV are located there and 68.8% of AIDS-related deaths in 2020 occurred in this region [6]. However, the prevalence of HIV in WA is much lower compared to that in southern Africa. This prevalence appears to be heterogeneous with less than 1% being located in Senegal and Mauritania, and nearly 4% in Côte d’Ivoire [18,19]. Consequently, the prevalence of cryptococcal meningitis, which has received very little research attention, has been estimated to be between 2.49% and 7.8% in WA [20,21], with a very significant lethality linked to the unavailability of systemic antifungals [21,22]. Indeed, FLC, the most available SA in WA, does not reduce this lethality of cryptococcal meningitis despite the use of high doses of this molecule as monotherapy in Côte d’Ivoire [20]. In fact, the WHO recommends AMB deoxycholate (1 mg/kg/day) in combination with 5-FC (100 mg/kg/day) as a first-line treatment in the first week before using high-dose FLC (1200 mg/day) for the second week and at 800 mg/day from the third to the tenth week of treatment [23]. As an alternative, FLC (1200 mg/day) should be combined with 5-FC (100 mg/day) in case of unavailability of AMB and with AMB deoxycholate (1 mg/kg/day) in case of unavailability of 5-FC in the first two weeks, before the use of FLC alone (800 mg/day) from the third to the tenth week, followed then by treatment with 200 mg/day after the tenth week [23].
This example with cryptococcal meningitis also remains valid to a lesser degree with pneumocystosis caused by Pneumocystis jirovecii, an opportunistic fungus in commensalism in human lungs and which can cause acute asphyxiant pneumonia with a mortality rate of up to 80% in some cases [24]. However, fortunately, the treatment involves an available antibiotic, Cotrimoxazole. This is also the case for the American histoplasmosis caused by Histoplasma capsulatum var. capsulatum, an opportunistic fungus that seems to be underestimated in sub-Saharan Africa due to the lack of suitable diagnosis. In fact, H. capsulatum var. capsulatum is increasingly discovered in patients from Africa [25]. Its treatment requires ITC or AMB via injection in its deoxycholate or liposomal form [16]. Invasive candidiasis, mainly due to Candida albicans, a yeast very present in the environment but also in the digestive tract of a large majority of the population and aspergillosis due to fungal species ubiquitous in the environment (foremost among which is Aspergillus fumigatus), can also be added to this non-exhaustive list of fungal infections that require the availability of SAs [24]. The management of these last two examples requires the use of VRC [16], a molecule that is still unavailable in WA, and in case of failure of triazoles, the use of echinocandins, which are of another level.
This comparative unavailability of SAs in WA could be related to the relatively low frequency of IFIs—apart from cryptococcosis—that is in turn attributable to the weakness of the technical materials and the use of slightly sensitive biological tools of diagnosis [1,26]. Therefore, this situation requires an awareness of the health authorities to capacitate the medical biology laboratories but also to revise the curricula of the medical training for a consciousness of the practitioners. Likewise, it will be necessary to draw attention to the real needs for SA drugs, and to maintain moderate prices of these drugs which are often used in long-term treatment [27].
Fortunately, in NA, the situation seems to be more favorable according to data from Morocco and Tunisia, where even these echinocandins, last resort molecules, are already available on the market. This situation could be due to the fact that the diagnosis of IFIs is more established than in NA, where it is not negligible [28]. In Morocco for example, from 2014 to 2018, 617 cases of candidaemia were diagnosed at the Ibn Rochd University Hospital in Casablanca involving a large number of non-albicans Candida species including C. tropicalis (28%), C. parapsilosis (18%), C. glabrata (6%) and C. krusei (2%). These last two species are known for their reduced sensitivities to antifungals, in particular to azoles such as FLC. Candida krusei presents a natural resistance to FLC, while C. glabrata has a dose-dependent sensitivity to azoles [29]. This is also the case for aspergillosis, particularly the chronic form [30,31,32], one of the main risk factors of which is tuberculosis, a disease widely distributed in the studied regions [33]. Similarly, in Tunisia, these same IFIs are often mentioned and documented with a prevalence of 27.6% for aspergillosis (of which 15.2% is probable and 12.4% is possible) in Sfax between December 2004 and September 2007 [34], while in Sousse, a prevalence of 12% of invasive aspergillosis was noted (9.8% probable and 2% possible) from December 2009 to November 2011 [35]. Apart from aspergillosis, mucormycosis is also reported [36,37], as are other IFIs considered endemic in North America, such as blastomycosis, with at least two cases reported [38,39].
In addition to the availability of SAs, Tunisia has classified AMB as being on the top of the list of products to be prioritized. This demonstrates the Tunisian authorities’ awareness of the burden of IFIs.
This study presents certain limitations. First, the professional directory used to find the contact of managers in different countries may no longer be relevant because the staff is often changing. Second, data sources were different in particular between WA and NA. Third, on-site availability, notably in NA, does not always means physical availability in pharmacies and accessibility for patients, though the management of drug suppliers—which provide pharmacies—is also under the control of the pharmacy and drug directorates in NA.
5. Conclusion
Given the above limitations, NA is ahead of WA as regards the burden of IFIs both diagnostically and in the availability of SAs. The studies on the epidemiology of IFIs in Africa, particularly in WA, are limited. The partial data available highlights that IFIs are not negligible, though these may be underestimated. Thus, a network based on the directory of African pharmacy and drug directorates must be set up and run in collaboration with the national laboratory directorates for the harmonization of procedures in order to reduce morbidity and mortality related to IFIs. Once again, this is based on the desire, at all decision-making levels, to give the concerned actors the means to know, recognize, and appropriately deal with IFIs.
Author Contributions
Conception, design of the study and drafting of the article: K.D.; Data collection: K.D.; A.J-S.M.; O.S.; Reviewing the article: A.J-S.M.; M.A.D.; M.C.S.; M.N.; A.S.B.; D.N.; Final approval of the version to be submitted: All authors.
Data Availability
Data used to support the findings of this study are included within the article.
Acknowledgments
The authors are grateful to Dr Othmani in Tunisia.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Ethical Statement
Our study did not require an ethical board approval because it did not contain human or animal trials. However, a publication consent was obtained from the pharmacies and wholesalers.
References
- Diongue, K.; Diallo, M.A.; Seck, M.C.; Ndiaye, M.; Badiane, A.S.; Ndiaye, D. The evidence for unavailability of systemic antifungals in Senegal. Ther. Adv. Infect. Dis. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Netea, M.G. Medical mycology and fungal immunology: New research perspectives addressing a major world health challenge. Philos. Trans. R. Soc. B 2016, 371, 20150462. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell. 2020, 7, 143–145. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 21 February 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Epidemiological Estimates 2021; UNAIDS: Geneva, Switszerland, 2021; pp. 4–38. Available online: https://aidsinfo.unaids.org/ (accessed on 31 March 2023).
- World Health Organization (WHO). Global Tuberculosis Report; WHO: Geneva, Switszerland, 2022; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 31 March 2023).
- World Health Organization (WHO). Novel Coronavirus (COVID-19) Situation Dashboard; WHO: Geneva, Switszerland, 2021; Available online: https://www.who.int/ (accessed on 29 May 2021).
- ePILLY trop 2016: Maladies infectieuses tropicales. Edition web mise à jour Août 2016; Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT). Eds Alinéa Plus. 2016. Available online: https://www.infectiologie.com (accessed on 4 July 2021).
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Bruggerman, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Antifungal agents. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 140–159. [Google Scholar]
- Ministère de la Santé et de l’action Sociale (MSAS). Liste nationale de médicaments et produits essentiels du Sénégal. Arrêté ministériel N° 016917/MSAS/DGS/DPM. MSAS. 2018. Available online: https://www.dirpharm.net/images/sampledata/pdf/Liste_nationale.pdf (accessed on 28 January 2021).
- Direction de la Pharmacie et des Laboratoires (DPL) de la de Mauritanie. Liste Nationale des Médicaments Essentiels par niveau de soins; DPL: Nouakchott, Mauritania, 2007; Available online: http://digicollection.org/hss/documents/s17813fr/s17813fr.pdf (accessed on 29 May 2021).
- Ministère de la santé et de l’hygiène publique de la Côte d’Ivoire (MSHP). Index pharmaceutique 2019-002. MSLS. 2019. Available online: http://www.npsp.ci/INDEX-PHARMACEUTIQUE-EDITION-2019-VF.pdf (accessed on 31 March 2023).
- Organisation Mondiale de la Santé (OMS). OMS. 2021. Available online: https://www.afro.who.int/fr/countries/senegal (accessed on 29 May 2021).
- World Health Organization (WHO). Model List of Essential Medicines, 21st List, 2019; WHO: Geneva, Switszerland, 2019; Available online: http://www.who.int/ (accessed on 29 May 2021).
- Chabasse, D.; Pihet, M.; Bouchara, J-P. Émergence de nouveaux champignons pathogènes en médecine: Revue Générale. Rev. Fra. Lab. 2009, 2009, 71–86. [Google Scholar] [CrossRef]
- Programme commun des Nations Unies sur le VIH/sida (ONUSIDA). Regard sur l’épidémie du VIH en Afrique francophone. ONUSIDA. 2009. Available online: https://www.unaids.org/sites/default/files/media_asset/jc2031_conference_francophonie_fr_0.pdf (accessed on 29 May 2021).
- Ministère de la Santé et des Affaires Sociales de la Mauritanie (MSAS). Formation sur la Prévention de la Transmission Mère-Enfant (PTME) du VIH Manuel du Participant. MSAS. 2011. Available online: https://www.sante.gov.mr/?wpfb_dl=68 (accessed on 29 May 2021). [Google Scholar]
- Kouakou, G.A.; Ello, N.F.; Kassi, N.A.; Keita, M.; Doumbia, A.; Mossou, C.; Kassi, F.K.; Tanon, A.; Ehui, E.; Eholié, S.P. Fluconazole 1200 mg ou 800 mg dans le traitement de la cryptococcose neuroméningée en Côte d’Ivoire. J. Mycol. Med. 2017, 27, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sow, D.; Tine, R.C.; Sylla, K.; Djiba, M.; Ndour, C.T.; Dieng, T.; Ndiaye, J.L.; Faye, B.; Ndiaye, D.; Gaye, O.; et al. Cryptococcal Meningitis in Senegal: Epidemiology, Laboratory Findings, Therapeutic and Outcome of Cases Diagnosed from 2004 to 2011. Mycopathologia 2013, 176, 443–449. [Google Scholar] [CrossRef]
- Diallo Mbaye, K.; Lakhe, N.A.; Sylla, K.; Dia Badiane, N.M.; Touré, D.; Ka, D.; Cisse Diallo, V.M.P.; Massaly, A.; Dieye, A.; Diop, A.; et al. Cryptococcose neuro-méningée: Mortalité et facteurs associés au décès chez les patients hospitalisés au service des Maladies Infectieuses du CHNU de Fann. Med. Afr. Noire 2018, 65, 77–84. [Google Scholar]
- World Health Organization (WHO). Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241550277 (accessed on 31 March 2023).
- Chandenier, J.; Desoubeaux, G. Epidemiological transition of mycosis diseases in sub-Saharan Africa: From surface to depth. Bull. Soc. Pathol. Exot. 2015, 108, 41–45. [Google Scholar] [CrossRef]
- Mandengue, C.E.; Ekeng, B.E.; Oladele, R.O. Disseminated Histoplasmosis; A Threat in Advanced HIV Disease Population in Sub-Saharan Africa? J. Adv. Med. Med. Res. 2021, 33, 115–144. [Google Scholar] [CrossRef]
- Dieng, Y.; Dieng, T.; Sow, D.; Wlouhou, S.; Sylla, K.; Tine, R.; Ndiaye, M.; Ndiaye, J.L.; Faye, B.; Faye, O.; et al. Diagnostic biologique de la pneumonie à Pneumocystis au centre hospitalier universitaire de Fann, Dakar, Sénégal. J. Mycol. Med. 2016, 26, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Grillot, R.; Ratsimbazafimahefa, H.M.R.; Ravelomampianina, H.S.N. Access to antifungal drugs in Madagascar. The situation in 2020. J. Mycol. Med. 2021, 31, 101120. [Google Scholar] [CrossRef]
- Osman, M.; Al Bikai, A.; Rafei, R.; Mallat, H.; Dabboussi, F.; Hamze, M. Update on invasive fungal infections in the Middle Eastern and North African region. Br. J. Microbiol. 2020, 51, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, W.; Dhar, R.; Ashkanani, H.; Gupta, M.; Rachel, C.; Khan, Z.U. Species spectrum and antifungal susceptibility profile of vaginal isolates of Candida in Kuwait. J. Mycol. Med. 2015, 25, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Benjelloun, H.; Zaghba, N.; Yassine, N.; Bakhatar, A.; Karkouri, M.; Ridai, M.; Ridai, M.; Bahloui, A. Chronic pulmonary aspergillosis: A frequent and potentially severe disease. Méd. Mal. Infect. 2015, 45, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Jabrane, M.; Skandour, D.; Rochdi, Y.; Nouri, H.; Aderdoui, L.; Raji, A.; Alaoui, C.; Fadili, W.; Laouad, I. Severe Forms of Aspergillosis in Patients on Hemodialysis. Saudi J. Kidney Dis. Transpl. 2016, 27, 174–176. [Google Scholar] [CrossRef]
- El Hakkouni, A.; Mansouri, N. Invasive pulmonary aspergillosis in a patient with human immunodeficiency virus (HIV). Pan Afr. Med. J. 2018, 31, 40. [Google Scholar] [CrossRef]
- Badiane, A.S.; Ndiaye, D.; Denning, D.W. Burden of fungal infections in Senegal. Mycoses 2015, 58, 63–69. [Google Scholar] [CrossRef]
- Hadrich, I.; Makni, F.; Sellami, H.; Cheikhrouhou, F.; Sellami, A.; Bouaziz, H.; Hdiji, S.; Elloumi, M.; Ayadi, A. Invasive aspergillosis: Epidemiology and environmental study in haematology patients (Sfax, Tunisia). Mycoses 2010, 53, 443–447. [Google Scholar] [CrossRef]
- Gheith, S.; Saghrouni, F.; Bannour, W.; Ben Youssef, Y.; Khelif, A.; Normand, A.C.; Said, M.B.; Piarroux, R.; Njah, M.; Ranque, S. Characteristics of Invasive Aspergillosis in Neutropenic Haematology Patients (Sousse, Tunisia). Mycopathologia 2014, 177, 281–289. [Google Scholar] [CrossRef]
- Zayet, S.; Zaghdoudi, A.; Ammari, L.; Kilani, B.; Tiouiri Benaissa, H. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection in a patient with diabetes mellitus: A case report. IDCases 2021, 23, e01022. [Google Scholar] [CrossRef]
- Zehani, A.; Smichi, I.; Chelly, I.; Marrakchi, J.; Besbes, G.; Haouet, S.; Kchir, N. Infection opportuniste agressive suite à une extraction dentaire chez un diabétique tunisien: Mucormycose rhinocérébrale agressive. Tunis Med. 2017, 95, 378–380. [Google Scholar] [PubMed]
- Rouhou, S.C.; Racil, H.; Ismail, O.; Trabelsi, S.; Zarrouk, M.; Chaouch, N.; Hantous, S.; Khaled, S.; El Mezni, F.; Chabbou, A. Pulmonary blastomycosis: A case from Africa. Sci. World J. 2008, 8, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.C.; Bachouch, I.; Belloumi, N.; Kacem, M.; Mlika, M.; El Mezni, F.; Fenniche, S. Blastomycose pulmonaire. Pan Afr. Med. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Map of Africa showing study sites with West African countries in yellow and North African countries in red.
Figure 1.
Map of Africa showing study sites with West African countries in yellow and North African countries in red.
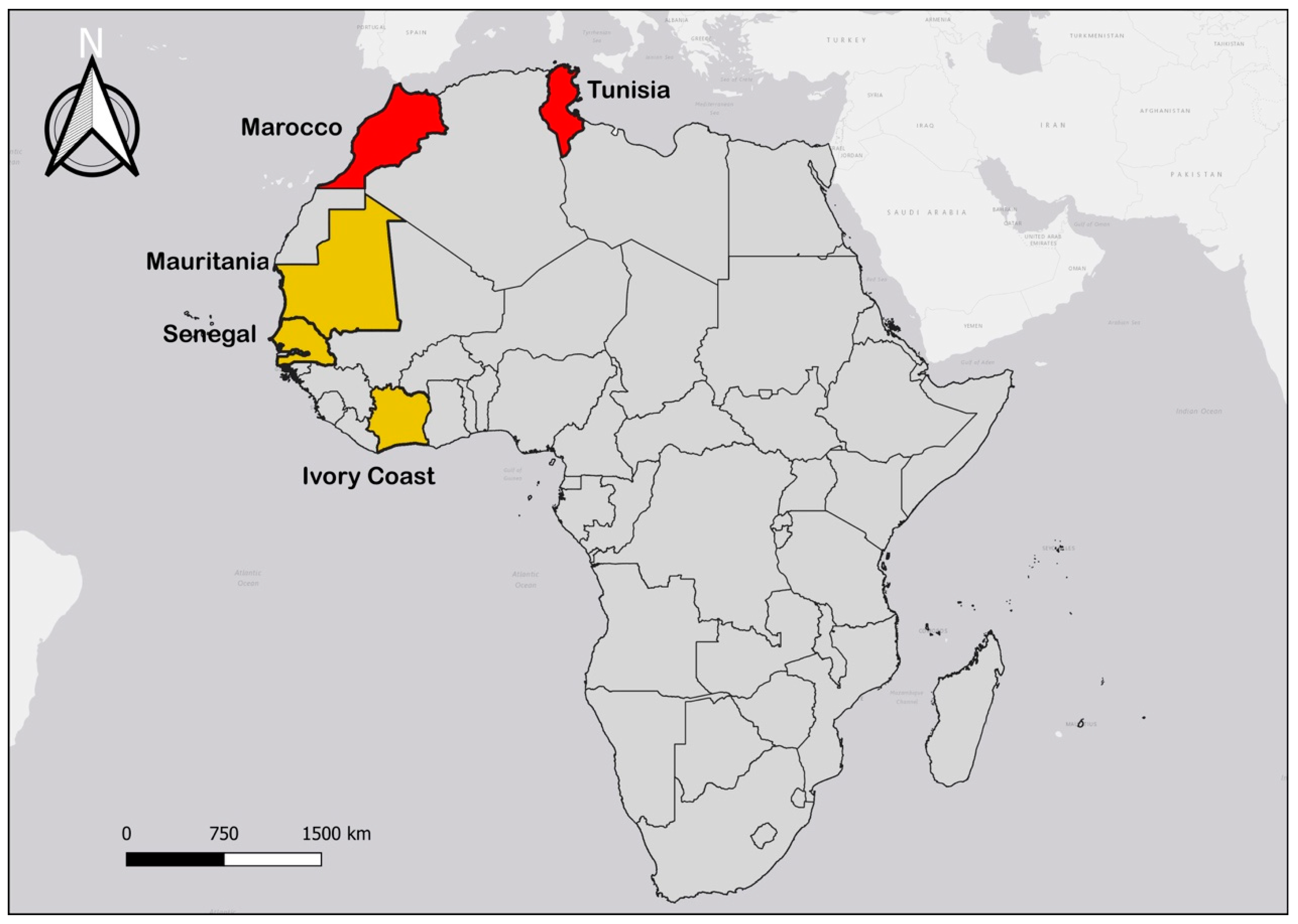
Table 1.
Profile of the study sites according to WHO [15].
Table 1.
Profile of the study sites according to WHO [15].
| Senegal | Mauritania | Ivory Coast | Morocco | Tunisia | |
|---|---|---|---|---|---|
| Population (x106) | 15,412 | 4,301 | 23,696 | 35,277 | 11,403 |
| GNI/inhabitant ($) | 2240 | 2850 | 2900 | 7000 | 10960 |
| Life expectancy (M/F) | 65/69 | 63/65 | 54/56 | 75/77 | 74/78 |
| TEH (in % of GDP) | 4.7 | 3.8 | 5.7 | 5.9 | 7.0 |
GNI: Gross National Income; M/F: Male/Female; TEH: Total Expenditure on Health; GDP: Gross Domestic Product.
Table 2.
Dosage and galenic form of systemic antifungal drugs available in West Africa: Côte d’Ivoire, Mauritania, and Senegal.
Table 2.
Dosage and galenic form of systemic antifungal drugs available in West Africa: Côte d’Ivoire, Mauritania, and Senegal.
| Classes | INN | Dosage | Galenic Forms | Availability | ||
|---|---|---|---|---|---|---|
| Côte d’Ivoire | Mauritania | Senegal | ||||
| Polyenes | AMB | 10% | Drinkable Susp. | + | - | - |
| 50 mg | Inj. Sol. Powder | + | - | - | ||
| 250 mg | Capsule | +* | - | - | ||
| Azoles | FLC | 50 mg | Capsule | + | + | + |
| 100 mg | Capsule | + | + | + | ||
| 150 mg | Capsule | + | + | + | ||
| 200 mg | Capsule | + | - | + | ||
| 2 mg/mL | Inj. Sol. | + | - | + | ||
| 2 mg/mL | IV Inf. Powder | - | + | + | ||
| 50 mg/5 mL | Drinkable Susp. | + | + | + | ||
| ITC | 100 mg | Capsule | + | - | - | |
* Available upon request; AMB: Amphotericin B; FLC: Fluconazole; Inf.: Infusion; Inj.: Injectable; INN: International Nonproprietary name; ITC: Itraconazole; IV: Intravenous; Perf.: Perfusion; Sol.: Solution; +: Available; -: Unavailable.
Table 3.
Systemic antifungals available in North Africa: Morocco and Tunisia.
| Classes | INN | Dosage | Galenic Forms | Availability | |
|---|---|---|---|---|---|
| Morocco | Tunisia | ||||
| Polyenes | AMB | 10 % | Drinkable Susp. | + | - |
| 50 mg | Inj. Sol. Powder | + | + | ||
| Azoles | FLC | 50 mg | Capsule | + | + |
| 100 mg | Capsule | - | + | ||
| 150 mg | Capsule | + | + | ||
| 200 mg | Capsule | + | + | ||
| 2 mg/mL | Inj. Sol. | + | + | ||
| 2 mg/ mL | IV Inf. Powder | + | + | ||
| 50 mg/5 mL | Drinkable Susp. | - | + | ||
| ITC | 100 mg | Capsule | - | + | |
| VRC | 50 mg | Tablet | + | + | |
| 200 mg | Tablet | + | + | ||
| 200 mg | IV Inf. Powder | + | + | ||
| 40 mg/mL | Drinkable Susp. | - | + | ||
| Echinocandins | CPF | 50 mg | Inj. Sol. Powder | - | + |
| 70 mg | Inj. Sol. Powder | - | + | ||
| MCF | 50 mg | IV Inf. Powder | + | - | |
| 100 mg | IV Inf. Powder | + | - | ||
| ANF | 100 mg | Inj. Sol. Powder | - | + | |
| 5-Flucytosine | 5-FC | 2.5 g/250 mL | IV Inf. Powder | + | - |
AMB: Amphotericin B; ANF: Anidulafungin; CPF: Caspofungin; FLC: Fluconazole; Inf.: Infusion; Inj.: Injectable; INN: International Nonproprietary name; ITC: Itraconazole; IV: Intravenous; MCF: Micafungine; Perf.: Perfusion; Sol.: Solution; VRC: Voriconazole; 5-FC: 5-Fluorocytosine.
© 2023 Copyright by Authors. Licensed as an open access article using a CC BY 4.0 license.


