Afr. J. Parasitol. Mycol. Entomol. 2024, 1(2), 2; doi:10.35995/ajpme2010002
Molecular detection of Wuchereria bancrofti, Loa loa and Mansonella perstans from dried blood spots taken from pregnant women in rural Burkina Faso
1
Clinical Research Unit of Nanoro, Nanoro, Burkina Faso
2
Institut de Recherche en Sciences de la Santé/Direction Régionale du Centre-Ouest, Bobo Dioulasso, Burkina Faso
3
Malaria and Neglected Tropical Diseases Laboratory, National Centre of Tropical Medicine, Biomedical Research Networking Center of Infectious Diseases (CIBERINFEC), Instituto de Salud Carlos III, 28029 Madrid, Spain
4
Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, 28040 Madrid, Spain
*
Corresponding author: tta@isciii.es; Tel.: +34-918-223-169
How to cite: Tahita, M.C., Ta-Tang, T.-H., Kaboré, B.; Capote-Morales, R., Molina de la Fuente, I.; Cruces, R., Ilboudo, H.; Sanon, E.D.G.; Ouedraogo, E.N.; Nana, B.; González, V.; Ouattara, D.; Sangara, D.; García, L.; Benito, A.; Tinto, H.; Berzosa, P. Molecular detection of Wuchereria bancrofti, Loa loa and Mansonella perstans from dried blood spots taken from pregnant women in rural Burkina Faso. Afr. J. Parasitol. Mycol. Entomol., 2024, 1(2): 2; doi:10.35995/ajpme2010002.
Received: 5 October 2023 / Accepted: 6 December 2023 / Published: 15 January 2024
Abstract
:Introduction: Human filariasis causes high morbidity and severe illness. There is a link between helminth infection and anemia. The objective of this study was to estimate the prevalence of blood-dwelling microfilariae among pregnant women in Burkina Faso using a molecular technique and attempt to find an association between anemia and filarial infection. Methods: A total of 1018 dried blood spot samples (DBS) were collected from pregnant women at the Health District of Nanoro. The DNA was isolated from DBS samples using a rapid and simple method. Afterward, the isolated DNA was assayed using the Filaria real-time PCR (F-RT-PCR) method. Results: Ten F-RT-PCR-positive samples were obtained as follows: two W. bancrofti (0.2%), four L. loa (0.39%), and four M. perstans (0.39%). No concomitant filarial infections were detected, as well as no coinfections between filarial disease and malaria. There was no link between the presence of W. bancrofti, L. loa, or M. perstans and anemia in pregnant women. Conclusions: The prevalence and intensity of human filariasis in this study were low for all of the samples in which microfilariae were detected. The F-RT-PCR can be a confirmatory test for diagnosis in remote areas due to its effectiveness in detecting and differentiating, both sensitively and specifically, a wide range of filarial parasites.
Keywords:
Wuchereria bancrofti; Loa loa; Mansonella perstans; dried blood spot; Burkina Faso; real-time PCR1. Introduction
Human filarial diseases are caused by parasitic roundworms whose progeny, the microfilariae, can be found in the peripheral blood or skin, depending on the filarial species [1]. To summarize, onchocerciasis or river blindness is caused by Onchocerca volvulus, and it is associated with vision impairment or blindness as well as severe dermatitis [2]. Lymphatic filariasis (LF) is caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori. LF patients suffer from lymphedema in their lower extremities, lymphedema in the scrotum (hydrocele), or tropical pulmonary eosinophilia, which is characterized by asthma-like symptoms. Loiasis, the eye-worm, is caused by Loa loa; its typical clinical signs are the subconjunctival migration of the adult worm, Calabar swellings, pruritus, oedema, and arthralgia. Mansonellosis is caused by the following three main species: Mansonella perstans, Mansonella ozzardi, and Mansonella streptocerca, normally with no specific symptoms [3,4,5]. These filarial nematodes have an important social and economic impact on the affected populations, causing high morbidity and serious illnesses resulting in social stigmatization, marginalization, and a loss of work for the afflicted [6].
The life cycles of filariae are similar. All filarial parasites are transmitted by the bite of a female blood-sucking arthropod or vector (mosquitoes, black flies, and deerflies) [1,3,7]. Third-stage larvae (L3) infect a new host when the vector feeds. They penetrate into the skin through the wound inflicted by the insect, L3 larvae, undergo two molts and eventually develop into sexually mature adult worms. Adult females are viviparous; they release thousands of microfilariae into the blood or skin, where they are picked up by vectors during their blood meal after being fertilized by adult male parasites. Ingested microfilariae undergo two molts to become infective L3 larvae that are transmitted to the human host during subsequent bloodmeals [1,3,4].
In 2000, The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched with the objective of eliminating this disease as a public health problem by 2020 [8]. The eradication of LF relies on mass drug administration (MDA) using the following three drugs that are currently available for treatment: diethylcarbamazine (DEC), albendazole, and ivermectin [6,9]. GPELF ended in 2020 without achieving the goal of globally eliminating LF by 2020. Since the start of GPELF, the number of infections has been reduced by 74% globally. The latest estimate is that 51.4 million people are infected [10,11]. The goal of the latest Neglected Tropical Diseases Roadmap 2021–2030 is to eliminate LF as a public health problem in 80% of endemic countries by 2030 [11].
Loa loa is endemic in eleven African countries based on the prevalence of eye worm history through a Rapid Assessment Procedure for Loiasis (RAPLOA) [12]. An estimated 14.4 million people live in areas with high infection rates. Another 15.2 million people live in areas where 20–40% of people are reported to have had past eye worm history [13]. In several endemic areas, it co-exists with onchocerciasis and LF, and this represents a public health issue because the ivermectin and DEC treatment administered during MDA programs against LF and ivermectin MDA for onchocerciasis can lead to severe adverse effects (SAEs), including fatal cases of encephalopathy, especially with a high L. loa microfilarial load [14,15].
Mansonellosis, the most benign human filariasis, remains a neglected human filarial infection despite the fact that 600 million people are at high risk of infection in Africa and parts of Central and South America [16,17,18]. Studies on M. perstans infections in Burkina Faso are scarce. Kyelem et al. [19] conducted a study to determine the impact of long-term ivermectin mass drug administration (MDA) on the W. bancrofti and M. perstans infections in Burkina Faso. In 2012, M. perstans microfilariae was observed in an atypical and exceptional location from the cervicovaginal smear of a patient from Mangodara (Burkina Faso) during cervical cancer screening [20]. To date, the incidence of mansonellosis in Burkina Faso is underestimated, and health authorities have paid little attention to this disease.
There is an association between helminth infection and anemia, and there is also the possibility that this host’s immune responses are modulated by the helminths infecting them [21]. Anemia in pregnancy has a very important socio-economic impact since it can adversely affect maternal and fetal well-being, resulting in increased morbidity and fetal death among populations with low socio-economic status [22,23].
Estimating the prevalence of blood-dwelling microfilariae among pregnant women in rural areas is of great interest as it can inform public health protocols. Therefore, this study was conducted to evaluate, through the use of molecular techniques, the prevalence of W. bancrofti, L. loa, and M. perstans microfilariae in pregnant women from some communities in Burkina Faso and to lay a foundation for future epidemiological and distribution studies of human filarial infection. Since the prevalence and intensity of human filariasis in Burkina Faso were expected to be low due to the MDA program, which intervened by implementing ivermectin and albendazole in this country, a technique with high sensitivity and specificity was required. For these reasons, the chosen technique was the Filaria real-time PCR (F-RT-PCR). Additionally, this study was an attempt to find an association between anemia in pregnant women and filarial infection.
2. Materials and Methods
2.1. Study area: brief description
The present study was carried out by the Clinical Research Unit of Nanoro (CRUN) in the Nanoro Health District (Figure 1). The Nanoro Health District is one of the seven (07) districts of the center-west health region. The district headquarters is in the Nanoro department, 90 kilometers from Ouagadougou, the capital city. This district’s area is 1302 km2 or 5.98% of the center-west region’s area of 21752 km2. This district is characterized by a high migration flux among youngsters toward the capital city and the bordering countries. The literacy rate is about 23% of the population. Since February 2009, the CRUN has implemented a health and demographic surveillance system (HDSS) involving 66,409 individuals (up to the year 2022).
2.2. Study design and eligibility criteria
Blood samples were collected from a cross-sectional study aiming to characterize the clinical epidemiology of pregnant women attending antenatal care (ANC) for the first time. For this reason, all pregnant women attending routine ANC visits at the Health District of Nanoro centers (Nanoro, Pella, Soaw, and Kindi) (Figure 1), regardless of their gestational age, were invited to participate in the study.
2.3. Study population and sampling
The targeted study population was pregnant women aged between 15 and 45 years old attending ANC at one of the recruiting sites during the study period. During the visit and after obtaining a written informed consent form, a standardized questionnaire was administered to assess socio-demographic data, followed by a physical examination conducted by the study nurses. From each patient, blood samples were collected for malaria rapid diagnostic tests (m-RDT) for prompt management of the study participant, including malaria microscopy and a dried blood spot (DBS). The collected DBS samples were stored in individual plastic bags at adequate temperatures and then transferred to the laboratory of the National Centre of Tropical Medicine (Madrid, Spain).
2.4. Sample size
A total of 1018 DBSs collected from pregnant women living in the recruitment area at the Health District of Nanoro were included in this study. They were collected in the framework of malaria projects from April 2010 to September 2010 and 10 years later, from December 2020 to March 2021.
2.5. Filarial DNA extraction
The DNA from DBS was extracted using a fast and simple non-enzymatic lysis method named Investigator STR GO! Lysis Buffer (QIAGEN GMBH, Germany). A punch 3 mm in diameter was collected from the center of the DBS using a handheld hole puncher. This 3 mm punch was immediately placed into a 1.5 mL tube before adding 100 µL of the lysis buffer, which was vortexed at high speed for 10 s and placed in the heat block at 95 °C for 2.5 min. Then, the tube was put in the centrifuge at full speed (13,000 rpm) for 2 min. The isolated DNA was stored at 4 °C or −20 °C for future analysis. Filter paper samples were handled according to safety procedures to prevent the contamination of samples by disinfecting the handle puncher in NaOH 5 M solution and rinsing the residual NaOH with distilled water.
2.6. Molecular analysis: Filaria Real-Time PCR (F-RT-PCR)
The presence of W. bancrofti, L. loa, and M. perstans in the DNA isolated from DBS samples was assayed using the Filaria real-time PCR (F-RT-PCR) at a final volume of 20 µL, as described by Ta-Tang et al. [24,25]. Briefly, F-RT-PCR was performed using Luna® Universal qPCR Master Mix 2x (New England Biolabs), and the amplification conditions were 1 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 15 s at 48 °C, 30 s at 60 °C and 2 s at 75 °C. All F-RT-PCR reactions were performed using a Rotor-Gene Q 5plex (QIAGEN GMBH, Germany). Clinical samples were run in duplicate and in parallel with appropriate positive and negative controls. Ultrapure water was used as the no-template control (NTC).
A sample was considered positive for filaria parasite if the melting temperature (Tm) curve of the amplified fragments was 76 °C ± 0.5 °C (Figure 2) and the species identification was conducted according to the amplified product size performed using automatic gel electrophoresis (QIAxcel Advanced, QIAGEN GMBH, Germany) or agarose gel electrophoresis stained with Pronasafe (Figure 3). The ITS1 sizes of 312, 301, and 286 base pairs indicated infection with M. perstans, W. bancrofti, and L. loa, respectively.
In addition, the quality of the DNA obtained was checked using human small subunit ribosomal RNA gene sequences as the internal positive control [26].
2.7. DNA sequencing and sequence analysis
The confirmation of the filarial species was performed by sequencing the amplified fragment using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) on an ABI PRISM 3700 DNA analyzer (Applied Biosystems, Massachusetts, USA). Previously, PCR products were purified using Speedtools PCR Clean Up Kit 250 rxns (Biotools, B&M Lab, SA, Madrid, Spain). All amplified products were sequenced twice in both directions, with forward and reverse primers. The sequences obtained were checked with the sequences database from GenBank of the National Center for Biotechnology Information (NCBI), whose accession numbers are as follows: M. ozzardi (EU272180), M. perstans (EU272181, EU272182, MN432520), M. mariae (AB362562), O. volvulus (EU272179), L. loa (EU272176) and W. bancrofti (EU272178). They were also aligned using CLUSTAL W [27].
3. Results
3.1. Study population characteristics
Overall, 1018 participants attending ANC were included in this study and underwent the F-RT-PCR diagnostic test. The socio-demographic characteristics of pregnant women (age, gestational period, educational status) are provided in Table 1. Almost 75% of the study participants had an age between 18 and 34 years old. The median age was 26 years old, within a range of 15–45 years old. More than 80% were in their second and third trimesters. The illiteracy rate was 70% (712/1018). Signs and symptoms suggestive of malaria were absent in most of the cases. Approximately 60.81% (619/1018) had anemia with an average hemoglobin level of 10.54 g/dL and a standard deviation of 1.8 (Table 1).
3.2. Molecular analysis: Filaria Real-Time PCR (F-RT-PCR)
Out of the 1018 samples examined from pregnant women, ten samples tested positive for microfilaria parasites using the F-RT-PCR method, representing 0.98% of the total women studied as follows: two tested positive for W. bancrofti (0.2%), four for L. loa (0.39%) and four for M. perstans (0.39%) (Table 2). No concomitant filarial infections were detected. In addition, none of the ten F-RT-PCR-positive samples were co-infected with malaria parasites when comparing the F-RT-PCR and malaria-microscopic results and considering microscopy as the gold standard method for malaria diagnosis. Most filarial-positive participants in this study were localized in the Nanoro and Pella areas.
Although blood smears were collected for the examination of malaria parasites, these stained blood smears could have also been used to visualize the microfilariae of W. bancrofti, L. Loa, or M. perstans. However, none of the microscopists screened the slides for microfilariae. Therefore, the corresponding microfilaremia via microscopy for this filarial parasite is unavailable. The threshold cycle or Ct value was used to estimate the relative concentration of filarial organisms per milliliter present in the DBS samples by comparing the obtained values to an appropriate standard curve derived from a series of dilutions of known filarial organism concentrations.
The relative microfilaria intensity for the ten F-RT-PCR-positive samples is described in Table 2. These relative quantifications represent, in general, low microfilaremia for any of the microfilariae detected. The microfilaremia of sample #3B could not be calculated. This sample had detectable M. perstans of genomic DNA via F-RT-PCR, but the microfilariae burden was too low to be estimated correctly using F-RT-PCR based on the standard curve; this sample likely had a smaller number of microfilariae than sample #154A. There was no possibility of obtaining a new DNA isolation from sample #3B due to the lack of blood samples for conducting a new calculation of the microfilaremia.
3.3. Sequencing and analysis of the F-RT-PCR-amplified fragments
The amplified products generated using F-RT-PCR were sequenced and compared to W. bancrofti, L. loa, and M. perstans 18S ribosomal RNA gene sequences deposited in the GenBank/NCBI. A BLAST search showed 100% identity with W. bancrofti, L. loa, and M. perstans sequences, thereby confirming that the ten positive patients were infected with blood filarial parasites. These sequences were also aligned using CLUSTAL W [27] to confirm the diagnosis and detect possible mutations; no mutation in the amplified fragment was detected.
4. Discussion
In tropical regions, the distribution of the filarial species W. bancrofti, L. loa, and M. perstans often overlaps, such as in Cameroon, Equatorial Guinea, or Gabon [28,29,30,31,32]. The prevalence of LF has decreased drastically in many health districts in Burkina Faso through the implementation of MDA campaigns that have applied albendazole and ivermectin since 2001 [33]. Infections via the filarial parasite L. loa mainly affect inhabitants of endemic areas; Burkina Faso is not a region associated with this infection, although travelers visiting these endemic regions can also become infected [34]. Studies on M. perstans infections in Burkina Faso are scarce. As of today, the incidence of mansonellosis in Burkina Faso is underestimated [19,35].
The finding of W. bancrofti, L. loa, and M. perstans in the studied area was an important discovery, as it is the first time that these infections have been evaluated in pregnant women in Burkina Faso, and therefore, in the Nanoro Health District, using a quantitative real-time PCR as the diagnostic method. Previous studies related to W. bancrofti were performed using a cross-sectional descriptive survey, the Filariasis Test Strip (FTS), Binax ICT (Binax, Portland, ME), or microscopy, but not molecular methods for the quantitative detection of the causative agent of LF [19,33].
Just two pregnant women were infected with W. bancrofti, which represents a prevalence of 0.20%, and the low microfilaria load (114 and 200 microfilariae/mL) was concordant with the study conducted by Kima et al., 2019, in which the overall prevalence of the microfilaremia was 0.62%, and the average microfilaremia density was 106 microfilariae/mL [33]. It was not a surprise that both LF-positive samples belonged to the collected period of 2010 and that none of these samples from 2020 to 2021 were positive. In the first period, the MDA campaign, with the distribution of albendazole and ivermectin, had not been established for even ten years, whereas in the second period, the distribution had already been consistent for over fifteen years [36]. This result confirms the success of the MDA program [11].
Loiasis is typically found in heavily forested areas of west-central Africa, with a limited geographical distribution determined by the vector, a tabanid fly of the genus Chrysops spp. [37]. Burkina Faso is not an endemic area known for L. loa; therefore, it is possible that the four cases of loiasis detected in this study were from women who had previously lived in L. loa-endemic areas before becoming married or women who became infected from traveling there, and whose diagnoses were only possible to achieve via the molecular analysis of microfilariae [38,39]. Since three out of the four L. loa-positive samples were from the period 2020–2021, this indicates a recently acquired infection. Regarding the L. loa-microfilariae intensity, this study showed a low-to-moderate microfilaria load in all cases, according to the parasitological indicators described by other authors [15,40].
Mansonellosis infection is frequently missed due to its tiny size or misidentification during slide examination, which, together with the low microfilaremia, explains why it has received limited attention [17,41,42]. The prevalence of M. perstans in this present study, despite appearing very low (0.39%), indicates that authorities should pay more attention to this non-pathogenic filariasis and that it is time to place this neglected tropical disease on the Ministry of Public Health’s agenda and take it into consideration similar to other human filariasis. M. perstans filariasis was found to affect all segments of the population, independently of sex and age, affecting both school-age children and adults [43]. This study demonstrates that M. perstans is also present in Burkina Faso and backs up prior research in other countries [17,43]. The WHO’s MDA program, which includes DEC for LF and ivermectin for onchocerciasis, is expected to clear M. perstans microfilariae [11]. The reality, however, is that none of these treatments efficiently clear M. perstans parasitemia, nor do they have an impact on its transmission [17]. This explains the four M. perstans-positive samples obtained in this study, both in the year 2010 and in the years 2020–2021. As of today, Burkina Faso is not widely recognized as an endemic area for M. perstans [44].
Currently, there are few data reporting filarial infections in pregnant women. Malaria should not be the only study target for routine public health interventions during pregnancy, but also human filariasis. The ten filarial-positive cases detected in this study did not allow a reliable association between W. bancrofti, L. loa, or M. perstans with anemia to be established in pregnant women. However, it would be unfair to completely rule them out as possible contributing factors to the anemia observed in these pregnant women, especially when the most frequent anemia cause, malaria disease, was absent.
It is well known that molecular methods are much more sensitive for the detection of microfilariae than microscopic examinations [45,46,47]. In the present study, a quantitative real-time PCR developed by Ta-Tang et al. [24] was used. Only ten F-RT-PCR-filaria-positive samples were obtained, and although it is unlikely that this finding has an impact on the epidemiology of the disease, it shows that W. bancrofti, L. loa, and M. perstans are present in this region. In addition, a periodic update of the filariae geographical distribution in Burkina Faso is necessary because of the spontaneous or induced disappearance of certain foci and the emergence of other foci as a result of the WHO’s MDA program or migrations of infected populations from other endemic areas [11].
Unfortunately, some of the malaria microscopy slides (year 2010) cannot be re-examined in order to find microfilariae. In these cases, F-RT-PCR was found to be very interesting and useful for the retrospective study of human filariasis in this area using long-term stored samples and calculating an approximated microfilaremia [48]. There is a good correlation between the extent of microfilaremia predicted by F-RT-PCR and the level confirmed by microscopy [24,25]. The low parasite densities obtained and their low prevalence indicate that the population of the study is under the 1% endemicity threshold for W. bancrofti, L. loa, and M. perstans. Moreover, it reflects, on the one hand, the success of the WHO’s programs against LF and, on the other hand, the little abundance of infected vectors in those areas. However, further entomological investigations are needed to determine the real prevalence of infected vector transmitters of human filariasis.
The F-RT-PCR used in this study allowed the identification and differentiation of these filariae in the DBS samples. According to the Road Map for Neglected Tropical Diseases 2021–2030 [11], the actions required by the WHO to meet their 2030 targets include devising confirmatory diagnostic tests for use in low-prevalence settings that can assist with mapping, MDA-stopping decisions, and surveillance. The WHO also encourages the development and use of multiplex tests, rather than single tests, which can detect only a filarial species. Furthermore, the diagnostic method developed does not have to cross-react with L. loa and can be used both in vectors and in humans. The F-RT-PCR assay proposed in the present study is a pan-filarial molecular assay that is able to detect different human filariae in the same reaction with high sensitivity and specificity. In addition, it can be applied to clinical samples from any origin (blood, skin biopsies, organic fluids), and it is valid in both humans and vectors [24,45].
In addition, given the easy storage conditions and long-term storage capacity of the DBS, this method can be standardized for surveillance studies in endemic regions [49]. In this context, DBS collected over the years represented a precious filarial parasite DNA source for this study. The blood samples collected as DBSs is a method that is simple, practicable, has low-cost, is easy to transport and store. It should be a universal method for sample collection in epidemiological investigations and field trials [50,51].
5. Conclusion
The prevalence of human filariasis demonstrated in our study was low for any of the microfilariae detected. The progress achieved through the MDA program should be maintained by continuously monitoring these regions, anticipating a resurgence in LF, and ensuring the detection of LF cases with field-reliable diagnostic tests. The F-RT-PCR can be a confirmatory test for diagnosis in remote areas due to its capacity to detect and differentiate, both sensitively and specifically, a wide range of filarial parasites. In addition, further research should be conducted to determine the epidemiological impact of M. perstans among the country’s population and the implications that such results could have for the country’s health policy in relation to this disease and filarial disease control. Given the low number of filarial-positive samples, a reliable association between anemia and filariasis diseases cannot be established. Finally, DBS represents a cheap and easily accessible source of genetic material, not only for filarial DNA but also for other kinds of microorganisms for retrospective molecular analysis.
Limitations of the study
The present study was conducted only in pregnant women and, therefore, cannot be generalized to the whole population. To ascertain that the LF transmission is still occurring in BF, children under 10 years old should be included in future studies. Additionally, the lack of blood available did not allow the F-RT-PCR to be repeated, as was the case for sample #3B. However, these results are of utmost importance for confirming the presence of W. bancrofti, L. loa, and M. perstans in the studied area, even at low prevalence levels.
Author Contributions
Conceptualization, M.C.T., P.B., and T.-H.T.-T.; methodology, M.C.T. and T.-H.T.-T.; software, D.O., D.S., T.-H.T.-T., and R.C.-M.; validation, B.K., H.I., and E.D.G.S.; formal analysis, T.-H.T.-T.; investigation, M.C.T.; resources, H.T. and A.B.; data curation, I.M.d.l.F., R.C-M., and T.-H.T.-T.; writing—original draft preparation, T.-H.T.-T. and M.C.T.; writing—review and editing, T.-H.T.-T., M.C.T., P.B., and A.B.; visualization, E.N.O., B.N., R.C., V.G., and L.G.; supervision, M.C.T. and B.K.; project administration, M.C.T. and B.K.; funding acquisition, M.C.T. and H.T. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This study could not have been undertaken without the generosity of the pregnant women who were involved by donating their blood samples. We would like to express our special thanks to them. Additionally, we would like to acknowledge the support we received from Foundation Mérieux through their small grants programme.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and was ethically approved by the Institutional Ethic Committee of Centre Muraz (registration no. 005- 2010/CE-CM) and the Institutional Review Board of the Institut de Recherche en Science de la Santé, Burkina Faso (N/REF. A15-2020 CEIRES). Following an explanation of this study’s purpose, benefits, and potential risks, pregnant women attending the maternity ward provided written consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. Written informed consent was obtained from the patients to publish this paper.
Conflicts of Interest
The authors report no conflicts of interest in this work. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Nanduri, J.; Kazura, J.W. Clinical and laboratory aspects of filariasis. Clin. Microbiol. Rev. 1989, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Crainey, J.L.; Medeiros, J.F.; Pessoa, F.A.C.; Luz, S.L.B. Onchocerciasis; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Walther, M.; Muller, R. Diagnosis of human filariases (except onchocerciasis). Adv. Parasitol. 2003, 53, 149–193. [Google Scholar]
- Alhassan, A.; Li, Z.; Poole, C.B.; Carlow, C.K. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ehrens, A.; Hoerauf, A.; Hübner, M.P. Eosinophils in filarial infections: Inducers of protection or pathology? Front. Immunol. 2022, 13, 983812. [Google Scholar] [CrossRef] [PubMed]
- Lustigman, S.; Grote, A.; Ghedin, E. The role of ‘omics’ in the quest to eliminate human filariasis. PLoS Negl. Trop. Dis. 2017, 11, e0005464. [Google Scholar] [CrossRef]
- Mendoza, N.; Li, A.; Gill, A.; Tyring, S. Filariasis: Diagnosis and treatment. Dermatol. Ther. 2009, 22, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Global Programme to Eliminate Lymphatic Filariasis: Progress Report. 2020. Available online: https://www.who.int/publications-detail-redirect/who-wer9641-497-508 (accessed 28 July 2022).
- Molyneux, D.H. Advancing toward the Elimination of Lymphatic Filariasis. N. Engl. J. Med. 2018, 379, 1871–1872. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2021. Global Programme to Eliminate Lymphatic Filariasis: Progress Report. 2021. Available online: https://www.who.int/publications-detail-redirect/who-wer9741-513-524 (accessed 4 July 2023).
- WHO. 2020. Available online: https://www.who.int/publications-detail-redirect/9789240010352 (accessed 12 November 2021).
- Molyneux, D.H. Filaria control and elimination: Diagnostic, monitoring and surveillance needs. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 338–341. [Google Scholar] [CrossRef]
- CDC C-C for DC and CDC—Loiasis—Epidemiology & Risk Factors. 2019. Available online: https://www.cdc.gov/parasites/loiasis/epi.html (accessed 9 January 2021).
- Knopp, S.; Steinmann, P.; Hatz, C.; Keiser, J.; Utzinger, J. Nematode infections: Filariases. Infect. Dis. Clin. N. Am. 2012, 26, 359–381. [Google Scholar] [CrossRef]
- Whittaker, C.; Walker, M.; Pion, S.D.; Chesnais, C.B.; Boussinesq, M.; Basáñez, M.G. The Population Biology and Transmission Dynamics of Loa loa. Trends Parasitol. 2018, 34, 335–350. [Google Scholar] [CrossRef]
- Simonsen, P.E.; Onapa, A.W.; Asio, S.M. Mansonella perstans filariasis in Africa. Acta Trop. 2011, 120 (Suppl. 1), S109–S120. [Google Scholar] [CrossRef] [PubMed]
- Ta-Tang, T.H.; Crainey, J.L.; Post, R.J.; Luz, S.L.; Rubio, J.M. Mansonellosis: Current perspectives. Res. Rep. Trop. Med. 2018, 9, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ta-Tang, T.H.; Luz, S.L.; Crainey, J.L.; Rubio, J.M. An Overview of the Management of Mansonellosis. Res. Rep. Trop. Med. 2021, 12, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kyelem, D.; Sanou, S.; Boatin, B.A.; Medlock, J.; Coulibaly, S.; Molyneux, D.H. Impact of long-term ivermectin (Mectizan) on Wuchereria bancrofti and Mansonella perstans infections in Burkina Faso: Strategic and policy implications. Ann. Trop. Med. Parasitol. 2003, 97, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Bamba, S.; Barro-Traoré, F.; Liance, M.; Da, O.; Sanou, C.; Guiguemdé, T.R. Mansonelliasis identified by a cervicovaginal smear at the university hospital center of Bobo-Dioulasso (Burkina Faso). Med. Sante Trop. 2012, 22, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Adegnika, A.A.; Ramharter, M.; Agnandji, S.T.; Ateba Ngoa, U.; Issifou, S.; Yazdanbahksh, M.; Kremsner, P.G. Epidemiology of parasitic co-infections during pregnancy in Lambaréné, Gabon. Trop. Med. Int. Health 2010, 15, 1204–1209. [Google Scholar] [CrossRef]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015, 36, 146–149. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Mzembe, G.; Moya, E.; Verhoef, H. Iron deficiency anaemia in sub-Saharan Africa: A review of current evidence and primary care recommendations for high-risk groups. Lancet Haematol. 2021, 8, e732–e743. [Google Scholar] [CrossRef]
- Ta-Tang, T.H.; Febrer-Sendra, B.; Berzosa, P.; Rubio, J.M.; Romay-Barja, M.; Ncogo, P.; Agudo, D.; Herrador, Z.; Fernández-Soto, P.; Muro, A.; et al. Comparison of three PCR-based methods to detect Loa loa and Mansonella perstans in long-term frozen storage dried blood spots. Trop. Med. Int. Health 2022, 27, 686–695. [Google Scholar] [CrossRef]
- Ta-Tang, T.H.; Berzosa, P.; Rubio, J.M.; Romay-Barja, M.; Ncogo, P.; Agudo, D.; Herrador, Z.; Cerrada-Gálvez, L.; Benito, A. Evaluation of LAMP for the diagnosis of Loa loa infection in dried blood spots compared to PCR-based assays and microscopy. Mem. Inst. Oswaldo Cruz 2022, 116, e210210. [Google Scholar] [CrossRef]
- Rubio, J.M.; Post, R.J.; van Leeuwen, W.D.; Henry, M.C.; Lindergard, G.; Hommel, M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: The semi-nested multiplex malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 2002, 96 (Suppl. 1), S199–S204. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Amvongo-Adjia, N.; Koudou, B.; Njouendou, A.J.; Chounna Ndongmo, P.W.; Kengne-Ouafo, J.A.; Datchoua-Poutcheu, F.R.; Fovennso, B.A.; Tayong, D.B.; Fombad, F.F.; et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS Negl. Trop. Dis. 2015, 9, e0004184. [Google Scholar] [CrossRef] [PubMed]
- Drame, P.M.; Montavon, C.; Pion, S.D.; Kubofcik, J.; Fay, M.P.; Nutman, T.B. Molecular Epidemiology of Blood-Borne Human Parasites in a Loa loa-, Mansonella perstans-, and Plasmodium falciparum-Endemic Region of Cameroon. Am. J. Trop. Med. Hyg. 2016, 94, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Pion, S.D.; Montavon, C.; Chesnais, C.B.; Kamgno, J.; Wanji, S.; Klion, A.D.; Nutman, T.B.; Boussinesq, M. Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia. Am. J. Trop. Med. Hyg. 2016, 95, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Puente, S.; Lago, M.; Subirats, M.; Sanz-Esteban, I.; Arsuaga, M.; Vicente, B.; Alonso-Sardon, M.; Belhassen-Garcia, M.; Muro, A. Imported Mansonella perstans infection in Spain. Infect. Dis. Poverty 2020, 9, 105. [Google Scholar] [CrossRef]
- Mbassi, F.-A.E.; Mombo-Ngoma, G.; Ndoumba, W.N.; Yovo, E.K.; Eberhardt, K.A.; Ekoka Mbassi, D.; Adegnika, A.A.; Agnandji, S.T.; Bouyou-Akotet, M.; Ramharter, M.; et al. Performance of Field’s Stain Compared with Conventional Giemsa Stain for the Rapid Detection of Blood Microfilariae in Gabon. Am. J. Trop. Med. Hyg. 2022, 107, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Kima, A.; Guiguemde, K.T.; Meda, Z.C.; Bougma, R.; Serme, M.; Bougouma, C.; Drabo, F. Evaluation of the effect of mass drug administration against lymphatic filariasis in three health districts and public health implications: Study of 12 epidemiological surveillance sites in Burkina Faso. Med. Sante Trop. 2019, 29, 55–60. [Google Scholar]
- Cobo, F. 7—Filariasis. In Imported Infectious Diseases; Cobo, F., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 91–114. [Google Scholar]
- Downes, B.L.; Jacobsen, K.H. A Systematic Review of the Epidemiology of Mansonelliasis. Afr. J. Infect. Dis. 2010, 4, 7–14. [Google Scholar] [CrossRef]
- Kima, A.; Guiguemde, K.T.; Serme, M.; Meda, Z.C.; Bougma, R.; Djiatsa, J.P.; Bougouma, C.; Drabo, F. Lymphatic Filariasis Transmission Assessment Survey in Burkina Faso in Connection with 4 Districts. Med. Trop. Sante Int. 2021, 1, mtsibulletin-n1. [Google Scholar] [CrossRef]
- Klion, A.; Nutman, T. Loiasis and Mansonella Infections. In Tropical Infectious Diseases; Churchill Livingstone: London, UK, 2006; pp. 1163–1175. [Google Scholar]
- Gobbi, F.; Postiglione, C.; Angheben, A.; Marocco, S.; Monteiro, G.; Buonfrate, D.; Mascarello, M.; Gobbo, M.; Boussinesq, M.; Bisoffi, Z. Imported loiasis in Italy: An analysis of 100 cases. Travel. Med. Infect. Dis. 2014, 12, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Priest, D.H.; Nutman, T.B. Loiasis in US Traveler Returning from Bioko Island, Equatorial Guinea, 2016. Emerg. Infect. Dis. 2017, 23, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Chounna Ndongmo, W.P.; Fombad, F.F.; Kengne-Ouafo, J.A.; Njouendou, A.J.; Longang Tchounkeu, Y.F.; Koudou, B.; Bockarie, M.; Fobi, G.; Roungou, J.B.; et al. Impact of repeated annual community directed treatment with ivermectin on loiasis parasitological indicators in Cameroon: Implications for onchocerciasis and lymphatic filariasis elimination in areas co-endemic with Loa loa in Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006750. [Google Scholar] [CrossRef] [PubMed]
- Ta-Tang, T.H.; Luz, S.L.; Merino, F.J.; de Fuentes, I.; López-Vélez, R.; Almeida, T.A.; Lanza, M.; Abrahim, C.M.; Rubio, J.M. Atypical Mansonella ozzardi Microfilariae from an Endemic Area of Brazilian Amazonia. Am. J. Trop. Med. Hyg. 2016, 95, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Crainey, J.L.; Costa, C.H.A.; de Oliveira Leles, L.F.; Vicente, A.C.P.; Muñoz, J.M.R.; Luz, S.L.B. Deep-sequencing reveals occult mansonellosis co-infections in residents from the Brazilian Amazon village of São Gabriel da Cachoeira. Clin. Infect. Dis. 2020; ahead of print. [Google Scholar] [CrossRef]
- Bassene, H.; Sambou, M.; Fenollar, F.; Clarke, S.; Djiba, S.; Mourembou, G.; L. Y., A.B.; Raoult, D.; Mediannikov, O. High Prevalence of Mansonella perstans Filariasis in Rural Senegal. Am. J. Trop. Med. Hyg. 2015, 93, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Rodari, P.; Salas-Coronas, J.; Bottieau, E.; Salvador, F.; Soriano-Pérez, M.J.; Cabeza-Barrera, M.I.; Van Esbroeck, M.; Treviño, B.; Buonfrate, D.; et al. A large case series of travel-related Mansonella perstans (vector-borne filarial nematode): A TropNet study in Europe. J. Travel. Med. 2022, 29, taac048. [Google Scholar] [CrossRef]
- Ta, T.H.; Moya, L.; Nguema, J.; Aparicio, P.; Miguel-Oteo, M.; Cenzual, G.; Canorea, I.; Lanza, M.; Benito, A.; Crainey, J.L.; et al. Geographical distribution and species identification of human filariasis and onchocerciasis in Bioko Island, Equatorial Guinea. Acta Trop. 2018, 180, 12–17. [Google Scholar] [CrossRef]
- Touré, F.S.; Bain, O.; Nerrienet, E.; Millet, P.; Wahl, G.; Toure, Y.; Doumbo, O.; Nicolas, L.; Georges, A.J.; McReynolds, L.A.; et al. Detection of Loa loa-specific DNA in blood from occult-infected individuals. Exp. Parasitol. 1997, 86, 163–170. [Google Scholar] [CrossRef]
- Nuchprayoon, S.; Junpee, A.; Poovorawan, Y.; Scott, A.L. Detection and differentiation of filarial parasites by universal primers and polymerase chain reaction-restriction fragment length polymorphism analysis. Am. J. Trop. Med. Hyg. 2005, 73, 895–900. [Google Scholar] [CrossRef]
- Yoboue, C.A.; Hosch, S.; Donfack, O.T.; Guirou, E.A.; Nlavo, B.M.; Ayekaba, M.O.; Guerra, C.; Phiri, W.P.; Garcia, G.A.; Schindler, T.; et al. Characterising co-infections with Plasmodium spp., Mansonella perstans or Loa loa in asymptomatic children, adults and elderly people living on Bioko Island using nucleic acids extracted from malaria rapid diagnostic tests. PLoS Negl. Trop. Dis. 2022, 16, e0009798. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, M.S.; Lin, M.; Dokomajilar, C.; Kemere, J.; Pilcher, C.D.; Dorsey, G.; Greenhouse, B. PCR-based pooling of dried blood spots for detection of malaria parasites: Optimization and application to a cohort of Ugandan children. J. Clin. Microbiol. 2010, 48, 3539–3543. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jaroensuk, J.; Leimanis, M.L.; Russell, B.; McGready, R.; Day, N.; Snounou, G.; Nosten, F.; Imwong, M. Long-term storage limits PCR-based analyses of malaria parasites in archival dried blood spots. Malar. J. 2012, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Tada, Y.; Sasai, K.; Baba, E. Improvement of DNA extraction method for dried blood spots and comparison of four PCR methods for detection of Babesia gibsoni (Asian genotype) infection in canine blood samples. J. Vet. Med. Sci. 2008, 70, 461–467. [Google Scholar] [CrossRef]
Figure 1.
Map of the study area Burkina Faso (in the right down square) showing the Health District of Nanoro and the location of the sampling health centers: Kindi, Nanoro, Pella, and Soaw.
Figure 1.
Map of the study area Burkina Faso (in the right down square) showing the Health District of Nanoro and the location of the sampling health centers: Kindi, Nanoro, Pella, and Soaw.
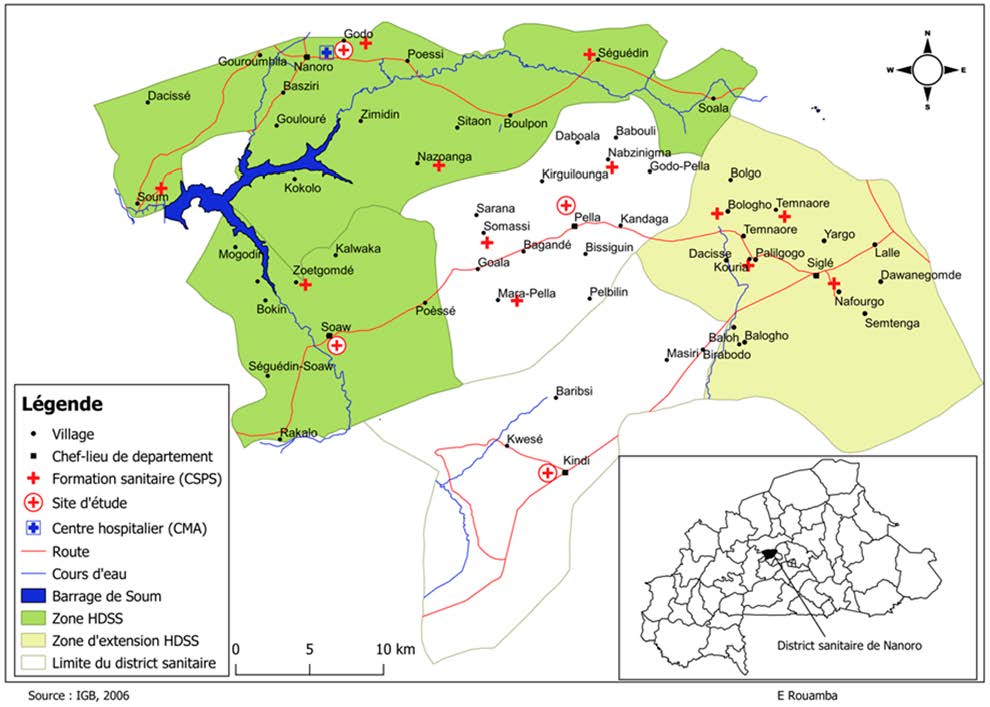
Figure 2.
Graphic showing the melting temperature (Tm) curve of the filarial-positive samples tested. The height of the Tm curve correlates with the microfilaria load of the samples. Note: The Rotor-Gene Q 5plex software gives the temperature decimals according to Spanish rules, using comma instead of period.
Figure 2.
Graphic showing the melting temperature (Tm) curve of the filarial-positive samples tested. The height of the Tm curve correlates with the microfilaria load of the samples. Note: The Rotor-Gene Q 5plex software gives the temperature decimals according to Spanish rules, using comma instead of period.
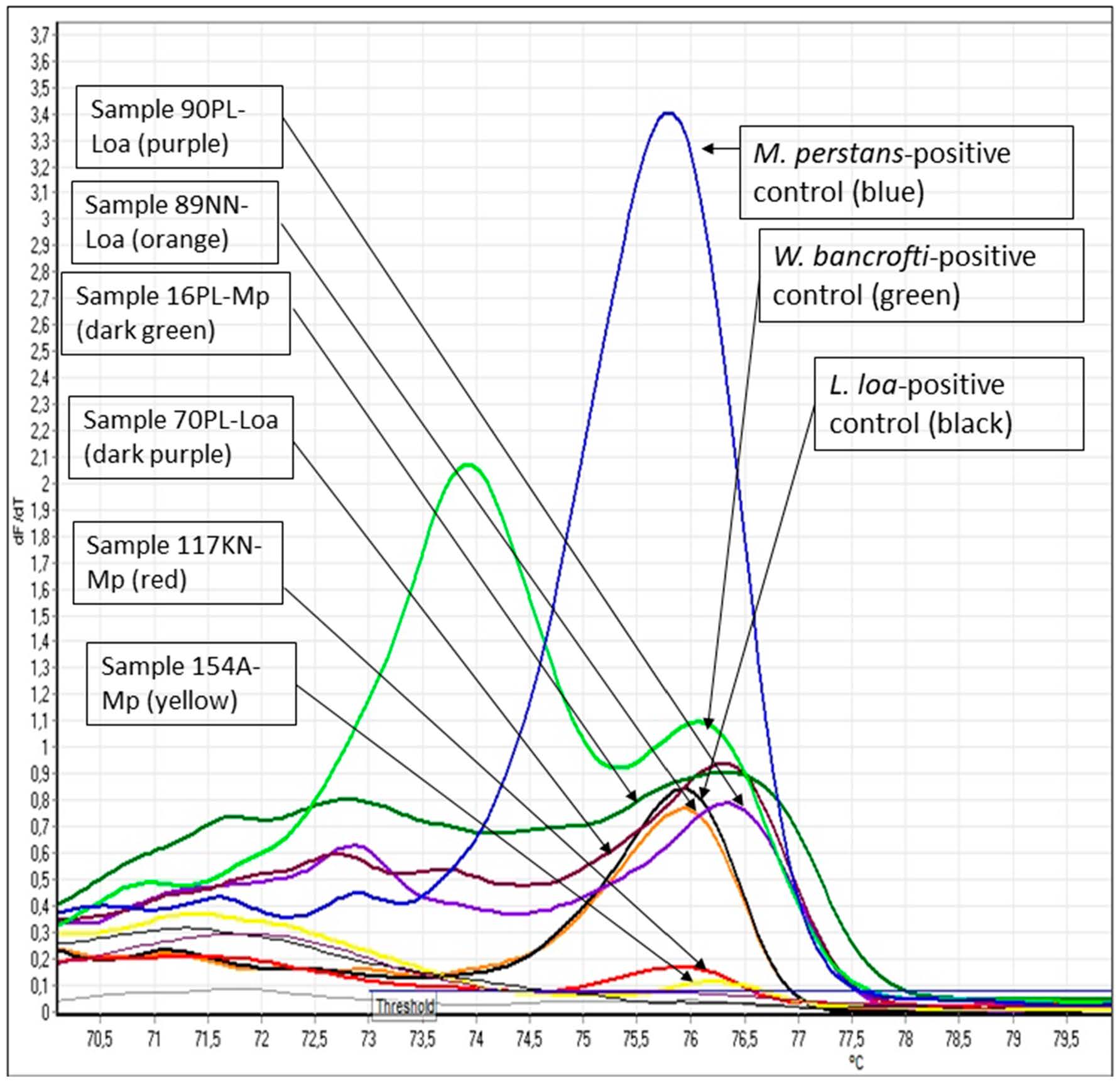
Figure 3.
Some of the F-RT-PCR-positive samples run in the agarose gel electrophoresis stained with Pronasafe (current name Condasafe. Condalab). The identification of the infecting filarial species was dependent on the size of the amplified fragment. Sizes of 312, 301, and 286 base pairs indicated infection with M. perstans, W. bancrofti, and L. loa, respectively. Note: the difference in the amplified size product was more significantly differentiated using sophisticated equipment with a higher resolution power, like QIAxcel capillary electrophoresis. Loa: L. loa; Mp: M. perstans; N: clinical samples negative for filarial disease: C-: negative control for any filarial parasite; LOA, WB, and MP: artificial positive controls created in the laboratory for L. loa, W. bancrofti, and M. perstans; NTC 1 and 2: non-template duplicated control. M: size marker 100 bp (Biotools, B&M Lab, SA).
Figure 3.
Some of the F-RT-PCR-positive samples run in the agarose gel electrophoresis stained with Pronasafe (current name Condasafe. Condalab). The identification of the infecting filarial species was dependent on the size of the amplified fragment. Sizes of 312, 301, and 286 base pairs indicated infection with M. perstans, W. bancrofti, and L. loa, respectively. Note: the difference in the amplified size product was more significantly differentiated using sophisticated equipment with a higher resolution power, like QIAxcel capillary electrophoresis. Loa: L. loa; Mp: M. perstans; N: clinical samples negative for filarial disease: C-: negative control for any filarial parasite; LOA, WB, and MP: artificial positive controls created in the laboratory for L. loa, W. bancrofti, and M. perstans; NTC 1 and 2: non-template duplicated control. M: size marker 100 bp (Biotools, B&M Lab, SA).

Table 1.
Socio-demographic characteristics of the pregnant women studied (n = 1018) attending antenatal care.
Table 1.
Socio-demographic characteristics of the pregnant women studied (n = 1018) attending antenatal care.
| Variables | n | % |
|---|---|---|
| Maternal age (years) | ||
| [15–18] | 65 | 6.39 |
| [19–34] | 824 | 80.94 |
| [34–35] | 129 | 12.67 |
| Gestational period | ||
| 1st trimester | 72 | 7.07 |
| 2nd trimester | 491 | 48.23 |
| 3rd trimester | 455 | 44.70 |
| Schooling | ||
| Yes | 190 | 18.66 |
| No | 828 | 81.34 |
| Parity | ||
| Nulliparous | 167 | 16.40 |
| 1–3 | 543 | 53.34 |
| ≥4 | 308 | 30.26 |
| ITN use | ||
| Yes | 762 | 74.85 |
| No | 162 | 15.92 |
| ND | 94 | 9.23 |
| Anemia | ||
| Yes | 619 | 60.81 |
| No | 399 | 39.19 |
ITN: insecticide-treated nets; ND: no data.
Table 2.
Characteristics of the ten F-RT-PCR-positive samples.
| Sample | Year | Age | District Health Center | m-RDT | Haemoglobin Level (g/dL) | F-RT-PCR | Microfilarial Intensity by F-RT-PCR (mF/mL) |
|---|---|---|---|---|---|---|---|
| 154A | 2010 | 38 | Nanoro | Negative | 10.8 | M. perstans | 85 |
| 170A | 2010 | 21 | Nanoro | Negative | 9.5 | L. loa | 100 |
| 3B | 2010 | 25 | Nanoro | Positive | 10.3 | M. perstans | NA |
| 138B | 2010 | 28 | Nazoanga | Negative | 9.5 | W. bancrofti | 114 |
| 197C | 2010 | 30 | Nanoro | Negative | 10.9 | W. bancrofti | 200 |
| 117KN | 2020_21 | 35 | Kindi | Negative | 11.8 | M. perstans | 574 |
| 89NN | 2020_21 | 25 | Nanoro | Negative | 10.1 | L. loa | 1180 |
| 16PL | 2020_21 | 39 | Pella | Negative | 9.2 | M. perstans | 725 |
| 70PL | 2020_21 | 24 | Pella | Negative | 7.3 | L. loa | 1205 |
| 90PL | 2020_21 | 24 | Pella | Negative | 7.7 | L. loa | 961 |
m-RDT: malaria rapid diagnostic test; F-RT-PCR: filaria real-time PCR; KN: Kindi; NN: Nanoro; PL: Pella; NA: not available; mF/mL: microfilariae per milliliter. The WHO considers anemia in pregnancy as hemoglobin values below 11 g/dL.
© 2024 Copyright by Authors. Licensed as an open access article using a CC BY 4.0 license.

