Afr. J. Parasitol. Mycol. Entomol. , 2(1), 3; doi:10.35995/ajpme2010003
Prevalence and schistosomiasis maintenance risk factors in the district of Bamako, Mali
1
Department of Epidemiology of Parasitic, Diseases, Faculty of Pharmacy, University of Sciences, Techniques and Technologies of Bamako, P.O. Box: 1805 Point G, Bamao, Mali
2
Centre de Recherche pour la lutte contre les Maladies Infectieuses Tropicales (CReMIT/TIDRC), Université d’Abomey-Calavi, Cotonou, Benin
3
Programme National de Lutte contre les Schistosomoses et les Géohelminthiases, BP: 99, Bamako, Mali
4
Institut National De Recherche En Santé Publique, BP: 1771, Bamako, Mali
*
Corresponding author: sdoumbo@icermali.org; Tél: +223-76039524
How to cite: Agniwo, P., Sidibé, B., Koné, A,, Akplogan, A., Guindo, H., Dossa, O,, Coulibaly, O., Dembélé, L., Traoré, M., Thera, M., Djimdé, A.A., Dabo, A., Doumbo, S.N. Prevalence and schistosomiasis maintenance risk factors in the district of Bamako, Mali. Priv. Pract. Infect. Dis., 2024, 2(1): 3; doi:10.35995/ajpme2010003.
Received: 2 October 2023 / Accepted: 1 February 2024 / Published: 28 February 2024
Abstract
:Introduction: Schistosomiasis is classically described as a rural parasitic infection affecting deprived communities. In Mali, where Schistosoma haematobium and S. mansoni are endemic, the disease is also spreading to urban areas. Our study aimed to describe the prevalence and schistosomiasis maintenance factors in the district of Bamako. Materials and methods: An observational cross-sectional study was carried out in April 2023 involving schoolchildren randomly selected aged 6 to 14 years from Taliko (a peri-urban area crossed by the Woyowayanko river known as an important breeding and infestation habitat of snails) and Missabougou (an area subjected to social and environmental changes, including the development of the canal serving the district). Data on sociodemographics, symptoms, and human–water contact activities were recorded through a structured questionnaire. Filtration and Kato–Katz techniques were used for S. haematobium and S. mansoni diagnosis. Overall, 736 urine samples and 668 stool samples were examined. Multivariate logistic analysis was employed to test for associations between variables. Results: The prevalence of S. haematobium was 18.2% [95% CI: 14.5–21.8%] and that of S. mansoni was 8.1% [95% CI: 4.2–11.9%]. This prevalence was significantly higher in Taliko than in Missabougou: 34.4% [95% CI: 29.1–39.6%] versus 2.4% [95% CI: 0–8.0%] (p = 0.001) for S. haematobium and 12.1% versus 4.3% for S. mansoni (p = 0.001). In Taliko, children frequenting the river were significantly more affected with S. haematobium and hematuria and had higher parasite intensity (p < 0.05). Children with macroscopic hematuria who attended a school close to the river (p = 0.018) in Taliko, as well as children who used to urinate in water, were more infected (p < 0.05). In Missabougou, no significant variation in human–water contact activities was recorded. Conclusion: Our results show that S. haematobium and S. mansoni were still rampant in the district of Bamako; they also highlight that the use of water from the Woyowayanko river appears to be the major risk factor for the maintenance of schistosomiasis in the district of Bamako.
Keywords:
Schistosomiasis; prevalence; Woyowayanko river; environmental changes; Bamako; Mali1. Introduction
Schistosomiasis is a parasitic disease caused by dioecious digeneans of the genus Schistosoma. It is a water–dependent disease of considerable medical and veterinary importance in tropical and subtropical regions in terms of prevalence. It is estimated that more than 236.6 million people in 78 countries required preventive treatment in 2019 [1]. Sub–Saharan Africa’s contribution to schistosomiasis prevalence accounts for 90% of cases [2].
In Mali, there are two major human–influenced species of schistosomes: S. haematobium and S. mansoni, which thrive around large artificial water supply structures (Markala, Selingue, and Manantali dams), small reservoirs on the Dogon plateau or around ponds, and in villages along the Senegal and Niger rivers. Therefore, schistosomiasis is endemic throughout the country [3,4,5]. While S. haematobium is the most common schistosome because of the wide–spread distribution of its intermediate host, Bulinus truncatus in all biotope types [6,7,8], S. mansoni distribution is rather focused on certain localities, such as the Office du Niger, Sélingué, and Baguinéda (Bamako) [3]. However, although it is a classically rural disease, urban areas are not spared from it either. Infections due to S. haematobium and S. mansoni have been reported in Bamako since 1951 [9]. Recently, prevalences of 14.7% and 1.5% have been recorded for S. haematobium and S. mansoni, respectively [10]. In response to schistosomiasis endemicity, Mali was one of the first countries in sub–Saharan Africa to set up the National Schistosomiasis and Soil–Transmitted Helminthiasis Control Programme (NSCP) in 1982. Thanks to its effectiveness and ease of implementation, mass drug distribution (MDD) with praziquantel (PZQ) for schistosomiasis has become an integral part of all schistosomiasis control programs. In Mali, the strategy was adopted in 2005, initially in highly endemic areas, before being extended to all other endemic zones. Despite the implementation of this program some twenty years ago, residual pockets have been reported in some areas of the country, such as the Senegal River Basin [11] and around Bamako [10,12]. In terms of epidemiology, social and environmental vulnerabilities contribute to the persistence and increase in schistosomiasis. The epidemiology of schistosomiasis is greatly influenced by ecological and human factors [13]. Ecological changes resulting from the presence of water points influence the survival, dispersal, distribution, and abundance of cercariae and host snails due to changes or modifications in habitat conditions. This is the case in the district of Bamako, which is crossed by numerous rivers such as Sogoniko, Bankoni, Farako, or Woyowayanko flowing from the Niger River. Elsewhere, other human factors likely to influence the epidemiology of schistosomiasis include urbanization. The role of urbanization in neglected tropical diseases (NTDs) has raised attention in the past decade because, in living memory, for the first time more people have lived in urban areas than in rural areas (56 and 44%, respectively), and, by 2050, this number is expected to reach 66% of the world population [14]. Even if this phenomenon is usually associated with economic progress and better social and health conditions, in developing countries, such progress is not synonymous with better living conditions [15]. In Bamako, for example, urbanization has transformed the quality of life of the inhabitants of the quarter of Missabougou, with the construction of the city’s third bridge, a modern hospital, an increase in the number of basic social services (schools, community health centers, road infrastructure, etc.), and also the development of the canal branching off from the River Niger and running alongside it as far as the village of Baguineda, 30 km away. Unlike Missabougou, in another neighborhood, Taliko, the persistence of the rural lifestyle, such as the use of surface water for domestic purposes (dishes, washing, pickling, etc.), added to poor sanitation created favorable conditions for the transmission of schistosomiasis. How do these changes affect the transmission of the disease in the two quarters of Bamako district, Missabougou and Taliko? The latest study to examine the impact of a change in the environment on transmission dates from 2010, where sanitation projects in the capital have made it possible to improve some of the city’s water, which are potential breeding sites for snails, the intermediate host of schistosomes [10]. This study aimed to describe sociodemographic and environmental risk factors for schistosomiasis among schoolchildren in two different settlements in the Bamako district. In addition, taking these results into account in local social project development could help break the schistosome transmission cycle.
2. Materials and methods
2.1. Study site
The study was carried out in two areas: one subjected to social and environmental changes (Missabougou) in the municipality VI (MVI) of Bamako, and a peri–urban area (Taliko) situated in the CIV of Bamako (Figure 1). The city covers an area of 1,420 km2 and is crossed by the Niger River and its tributaries. It belongs to the northern Sudanese climatic zone, with two main seasons. The rainy season extends from June to November, with thunderstorms and torrential rains at the beginning and end. Average annual rainfall is around 1,400 mm. The dry season extends from December to May. Temperatures are generally high, with an annual average of 33 °C.
In Taliko, a peri–urban area, Woyowayanko, a tributary of the Niger River crossing the village, was to be used as a rainwater collector. Over time, however, houses were built along and near the stream, which also serves to drain wastewater from the neighborhood (Figure 2A). The Taliko school is located not far from this river.
In Missabougou, the area has undergone many environmental changes, including the cleaning up of the canal running alongside the neighborhood, over which bridges have been built to make it easier for people to move from one side to the other (Figure 2B). The quarter is provided with a water supply. Located near the third bridge of the city, it has been equipped with a number of facilities, including a modern hospital, and is crossed by a large road of four lanes connecting it to the national road No. 6 (RN°6).
2.2. Type and period of the study
We carried out a cross–sectional study in April 2023 involving the collection and analysis of children’s urine and feces.
2.3. Study population
The target population was school–aged children (6 to 14 years old].
2.4. Sampling plan and sample size calculation
The sample size was calculated on the basis of the prevalence of S. haematobium (the most common schistosome species) at 70% from the precedent study [16]. The assumed precision of the prevalence rates to be measured was 6%, with an alpha risk of 5%. The Schwartz formula was used to calculate the minimum sample size. A minimum sample size of 322 was selected.
2.5. Procedures for gathering socio–demographic, socio–economic, paraclinical, and clinical data
Sociodemographic data (age, gender, and parent’s profession) and information on human–water contact activities, including type of water used (borehole, river, rain, drink), and the reason for visiting waterways (swimming, fetching water, taking a bath), were collected using a structured questionnaire. Information was obtained by two trained interviewers without knowledge of the individuals’ laboratory results. A clinical examination related to S. haematobium symptoms (bladder pains, pollakiuria, dysuria) was undertaken by a single physician without prior knowledge of the patients’ infection status. Macroscopic hematuria was determined with the naked eye; microscopic hematuria was determined using Hemastix strips (Ref: AZA – TESTUR10, SIEMENS medical solutions diagnosis).
2.6. Techniques and procedures for collecting parasitological data
For collecting urine and stool samples, each participant received two labeled specimen containers of 125 ml each and was asked to provide one fresh morning stool sample and one urine sample (taken between 10 a.m. and 2 p.m.). Urine samples were vigorously shaken, and 10 ml was filtered through a Whatman filter (CAT N° 1001–025, 25 mm). Filters were stained with a 3% ninhydrin solution, dried, and then examined under a microscope (×5 or ×10 magnification) to determine S. haematobium eggs [11]. The stool samples were examined using the standard Kato–Katz method for determining S. mansoni eggs under a microscope (×10 magnification). The intensity of S. haematobium infection was expressed as the number of eggs per 10 ml of urine and classified into three categories according to WHO classification: (i) no eggs; (ii) low (1–49 eggs per 10 ml of urine); and (iii) heavy (≥50 eggs per 10 ml of urine). S. mansoni intensity was expressed by eggs per gram of stool (epg) and then classified into four categories according to the WHO: (i) no eggs; (ii) low (1–99 epg); (iii) moderate (100–399 epg); and (iv) high (≥400 epg) [17]. Quality control was performed on 10% of randomly selected filters and slides, which were read by another experienced biologist. At the end of the study, all positive children were treated with PZQ (40 mg/kg body weight) in accordance with the National Schistosomiasis and Soil–Transmitted Helminthiasis Programme (NSCP).
2.7. Statistical analysis
Data were entered using Excel. Statistical analyses, such as prevalence calculations and intensity of infection, were performed using SPSS (IBM, version 23). Age was classified into two categories: 6–10 and 11–15 years. We performed multivariate logistic regression statistical analyses to assess the relationship between parasitic infections and demographic, sociocultural, and clinical factors. Differences in proportions were tested using the chi–square test or the exact Fisher test, depending on the data. P values below 0.05 were considered significant.
2.8. Ethics
The proposal was reviewed and approved by the Ethical Institutional Review Board (IRB) of the Faculty of Medicine and Dentistry and the Faculty of Pharmacy, University of Sciences, Techniques and Technologies of Bamako (N° 2023/69/CE/USSTB). Oral permission was obtained from school authorities and schoolchildren’s parents before starting the study. Free and informed consent was also obtained from the children. All participants who tested positive were treated with praziquantel (40 mg body weight after eating) following the recommendations of the National Schistosomiasis and Soil–Transmitted Control Programme of Mali.
3. Results
3.1. Prevalence and intensity of S. haematobium
The sex ratio in our study was 0.83 in favor of girls. The global prevalence of S. haematobium was 18.2% [95% CI 14.5–21.8]. The prevalence was significantly higher in Taliko than in Missabougou, and older children were more affected (p < 0.0001). However, infection did not vary significantly according to gender (p = 0.11). The intensity of infection also varied significantly among sites, age group, and gender, with all the heavy parasite loads described in Taliko and in females (p < 0.05) (Table 1).
The overall prevalence of S. mansoni was 8.1% [95% CI 4.2–11.9]. It was significantly higher in Taliko than in Missabougou (12.1 vs 4.3%). No high parasite load was observed at Missabougou for S. haematobium but only for S. mansoni (p < 0.0001). Older children were not more infected than younger ones (p =0.15); however, they were more heavily infected (p = 0.02) (Table 2).
3.2. Human–water contact activities associated with S. haematobium infection
Table 3 shows that children visiting the river were significantly more affected by urinary schistosomiasis and presented more microhematuria with lower parasite intensity in Taliko (p < 0.05). The source of water consumed by the children as well as the distance between the school and the river had no impact on parasitological parameters (p > 0.05). All children with macroscopic hematuria attended a school close to the river (p = 0.018). In addition, children who used to urinate in water were more infected (p < 0.05). No significant variation in human–water contact activities (frequentation of the river, domestic water source, distance from school to the river, or urine in water) was recorded in Missabougou (Table 3).
3.3. Sociodemographic parameters associated with S. haematobium infection
Table 4 shows the sociodemographic parameters associated with S. haematobium infection. The prevalence of microhematuria was significantly higher in older children (p = 0.038), and no female was highly infected in Taliko (p = 0.001). The prevalence of infection and hematuria was not associated with the parent’s occupation (p > 0.05) (Table 4).
3.4. Clinical signs associated with S. haematobium infection
In the peri–urban area, bladder pain and dysuria were significantly associated with microhematuria (p = 0.0001) and with S. haematobium infection (p < 0.05). No macroscopic hematuria was observed in Missabougou (Table 5).
4. Discussion
We recorded an overall prevalence of 34.4% versus 2.9% for S. haematobium and 12.07% versus 4.3% for S. mansoni for the peri–urban area (Taliko) and an area subjected to social and environmental changes (Missabougou), respectively. This prevalence of S. haematobium (18.2%) and S. mansoni (8.1%) was higher than that (14.7% and 1.5%) previously reported throughout the Bamako district by Dabo et al. (2015). Likewise, the prevalence of S. haematobium recorded in Taliko in MIV (34.4%) was slightly higher than that in 2015 (31.1%) in the same area but in April and November, respectively. This consistency or slight increase in prevalence indicates the persistence of the disease due to the active nature of the parasite within the population. Males in Taliko were significantly more heavily affected (p = 0.001). This result is similar to those described in the same area [10], but different from that reported by Agniwo et al. (2022) in the Kayes region of Mali. Globally, males were more heavily affected by S. haematobium than females (Table 1) (p = 0.003). The influence of gender on the prevalence or intensity of schistosomiasis is debatable and varies from one study to another. The results are sometimes higher in women, for example, in the Bandanyenje region (Zimbabwe) [18], and sometimes lower, such as in the Oromia region (Ethiopia) [19,20]. In the Kayes region (Mali) [21] or in southern Nigeria [22], there was no difference in disease prevalence between genders. The differences between genders in terms of prevalence or intensity could be explained more by behavioral differences (nature and intensity of water contact activities) than by immunological differences, according to a meta–analytical approach based on data from 23 schistosomiasis–endemic countries [23].
The high prevalence and intensity rates observed in Taliko compared to Missabougou would be mainly due to the various changes that have occurred or are underway in Missabougou (cf. Materials and Methods). The cleaning of the banks of the canal leads to the destruction of the habitat of snails (aquatic vegetation and debris of all kinds dumped into the bed). A similar experiment was conducted in another quarter of Bamako, Niomirambougou, in 2010 with the cleaning and development of the banks of the Farako River as part of the improvement of the living environment of the city of Bamako. Prior to intervention, the prevalence of urogenital and intestinal schistosomiasis and that of double infection were 89.8%, 30.2%, and 65%, respectively, in schools along the river [24]. Children frequenting the river had a higher prevalence of S. haematobium and presented more hematuria with a high parasite load in Taliko (p < 0.05). This result is opposed to that reported most recently by Agniwo et al. (2022) in the Kayes area, but is consistent with several other studies [10,25]. All children with macroscopic hematuria attended a school close to the river (p = 0.018). The short distance from habitats and schools favors access to contaminated water for children who easily engage in risky activities such as swimming and various practices. However, the influence of distance on schistosomiasis transmission is controversial. While some studies are in agreement with a positive association between short distance and infection [10,26,27], others argue the opposite [28]. We, however, believe that in addition to distance, some other factors, including sanitation in some communities like Taliko, where the river banks were used as garbage dumps, could have contributed to the higher level of infection. Children accustomed to urinating in water were more affected, with a higher load (p < 0.05). The fact that children were in contact with water for a long time, which means being exposed to cercariae for a long time, would be responsible for their infection. This factor not only exposes the children but is also a source of contamination of the water through the infected urine they simultaneously discharge into the water.
In our survey, sociodemographic parameters showed that children aged 11 to 14 in Taliko, the peri–urban area, had significantly more macroscopic hematuria than younger children (p < 0.0001). The great mobility of older children compared with the youngest children under parental control could explain this observation. We also noted that among children with bladder pain, pollakiuria, and dysuria, boys were significantly more affected in Taliko (p < 0.05). The same results were observed in Missabougou for pollakiuria and dysuria (p < 0.05). This observation supports several studies that have reported clinical signs in children in schistosomiasis–endemic areas [11,29,30].
Clinical and parasitological parameters revealed parasitemia in children presenting with macroscopic hematuria of high intensity. This finding is supported by several reports that show hematuria to be the pathognomonic paraclinical sign associated with the presence of S. haematobium eggs in urine [11,29,31]. Children with bladder pain were in the majority (p = 0.0001), in contrast to those with dysuria in Taliko, as recently reported in a study in the Kayes region, where these signs were reported in over 45% of affected children [11]. Overall, the prevalence of intestinal schistosomiasis was 8.1%, varying from 12.0% in Taliko to 4.3% in Missabougou. This means that, despite the environmental changes in Missabougou, especially the canal clean–up, transmission is still ongoing. In this case, there could be other sources of contamination apart from the canal. Further investigation will be useful to address this issue.
In terms of limitations, urine and stool analysis techniques are the gold–standard methods used in parasitological investigations. However, the sensitivity of these methods could be increased by increasing the number of urine samples and the number of Kato–Katz slides. On the other hand, an epidemiological study of schistosomiasis should always be accompanied by a malacological investigation to identify snail intermediate hosts and determine their natural infestation rates. Such a study could not be carried out because of the drying up of water points at the time of the survey, particularly in Taliko.
5. Conclusions
The study showed that both S. haematobium and S. mansoni still persist in the two study areas, especially in the peri–urban area, despite the annual mass drug distribution of praziquantel. The results show that the infection was more prevalent in Taliko (a peri–urban area) than in Missabougou (an area subjected to environmental changes). Visiting water points in Taliko for many human water contact activities, such as swimming for children, appears to be one of the most important risk factors for the maintenance of infection. In the absence of public swimming pools for children, cleaning the banks of water points could significantly reduce the risk of infection, in addition to providing health education and a proper supply of drinking water to the population.
Author Contributions
S.N.D., M.T., and A.D. participated in the study design. S.N.D., P.A., M.T., and A.D. updated the research methodology. S.N.D., P.A., M.T., A.D., L.D., B.J., A.A., H.G., O.D., and A.K. coordinated the trial. A.P. and S.N.D. carried out the statistical analysis. SND, P.A., and A.D. contributed to the drafting of the final document. S.N.D., P.A., M.A.T., A.D., L.D., A.A.D., A.K., and M.T. critically reviewed and approved the final manuscript before submission.
Acknowledgments
This article was produced by the WANETAM project, which is part of the EDCTP2 programme supported by the European Union (CSA2020–NoE–3130–WANETAM3). The authors would like to thank the principals of the various schools who helped us and the schoolchildren who provided the samples. We would also like to thank the staff of the MRTC (Malaria Research and Training Center), the staff of the Faculty of Medicine and Dentistry, and the Faculty of Pharmacy for providing the framework for manipulation.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- WHO. Schistosomiasis Fact Sheet. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 12 June 2023).
- Dawet, A.; Yakubu, D.; Longmut, R.; Benjamin, C.; Nannim, Y.; Daburum, N. Prevalence and intensity of Schistosoma haematobium among residents of Gwong and Kabong in Jos North Local Government Area, Plateau State, Nigeria. Int. J. Biol. Chem. Sci. 2012, 6, 1557–1565. [Google Scholar]
- Brinkmann, U.; Werler, C.; Traore, M.; Korte, R. The National Schistosomiasis Control Programme in Mali, objectives, organization, results. Trop. Med. Parasitol. 1988, 39, 157–161. [Google Scholar]
- Dabo, A.; Mahamat Badawi, H.; Bary, B.; Doumbo, O.K. Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasit. Vectors 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Traoré, M.; Maude, G.; Bradley, D. Schistosomiasis haematobia in Mali: Prevalence rate in school-age children as index of endemicity in the community. Trop. Med. Int. Heal. 1998, 3, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.; Coulibaly, G.; Furu, P. Distribution of freshwater snails in the river Niger basin in Mali with special reference to the intermediate hosts of schistosomes. Hydrobiologia 1987, 88, 77–88. [Google Scholar] [CrossRef]
- Coulibaly, G.; Madsen, H. Seasonal density fluctuations of intermediate hosts of schistosomes in two streams in Bamako, Mali. J. African Zool. 1990, 104, 201–212. [Google Scholar]
- Dabo, A.; Diop, S.; Doumbo, O. Distribution des mollusques hôtes intermédiaires des schistosomiases humaines à l’office du Niger (Mali). II: Rôle des différents habitats dans la transmission. Bull. la Société Pathol. Exot. 1994, 87, 164–169. [Google Scholar]
- Deschiens. The sanitary problem of bilharziasis in French Union countries. Bull. Exot. Pathol. Soc. 1951, 44, 350–377. [Google Scholar]
- Dabo, A.; Diarra, A.; Machault, V.; Kanté, A. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect. Dis. Poverty 2015, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Agniwo, P.; Sidibé, B.; Diakité, A.T.; Niaré, S.D.; Guindo, H.; Akplogan, A.; Ibikounlé, M.; Boissier, J.; Dabo, A. Ultrasound aspects and risk factors associated with urogenital schistosomiasis among primary school children in Mali. Infect. Dis. Poverty 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tandina, F.; Doumbo, S.; Koné, A.; Guindo, D.; Goita, S.; Sissoko, M. Épidémiologie des schistosomoses dans le village périurbain de Sotuba, dix années après la mise à échelle du traitement de masse au Mali. Médecine et Santé Tropicales 2016, 26, 51–56. [Google Scholar]
- Hunter, J.M. Inherited burden of disease: Agricultural dams and the persistence of bloody urine (Schistosomiasis hematobium) in the Upper East Region of Ghana, 1959–1997. Soc. Sci. Med. 2003, 56, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Nations, U. World urbanization prospects. United Nations Dep. Econ. Soc. Aff. 2014. Available online: https://www.un. org/en/development/desa/publications/2014–revision–world–urbanization–prospects.html (accessed on 12 June 2023). [Google Scholar]
- Hotez, P. Global urbanization and the neglected tropical diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005308. [Google Scholar] [CrossRef]
- LY, B.; Yaro, A.S.; Sodio, B.; Sacko, M. Persistance de la schistosomiase urinaire en zones endémiques soumises aux traitements de masse répétés au Mali. Int. J. Biol. Chem. Sci. 2019, 13, 369. [Google Scholar]
- WHO. Control of Schistosomiasis: Report of a WHO Expert Committee; Technical Report Series 728; WHO: Geneva, Switzerland, 1985. [Google Scholar]
- Chisango, T.J.; Ndlovu, B.; Vengesai, A.; Nhidza, A.F.; Sibanda, E.P.; Zhou, D.; Mutapi, F.; Mduluza, T. Benefits of annual chemotherapeutic control of schistosomiasis on the development of protective immunity. BMC Infect. Dis. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Kebede, T.; Bech, N.; Allienne, J.F.; Rey, O.; Erko, B.; Boissier, J. Genetic evidence for the role of non-human primates as reservoir hosts for human schistosomiasis. PLoS Negl. Trop. Dis. 2020, 14, e0008538. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, R.S.K. PCSK9 targets important for lipid metabolism. Clin. Res. Cardiol. Suppl. 2017, 12, 2–11. [Google Scholar]
- Dabo, A.; Diallo, M.; Agniwo, P.; Danté, S.; Traoré, A.; Diawaea, S.; Doucouré, B. Mass Drug Distribution Strategy Efficacy for Schistosomiasis Control in Mali (West Africa). Researcg Sq. 2021. [Google Scholar]
- Onyekwere, A.; Rey, O.; Nwanchor, M.C.; Alo, M.; Angora, E.K.; Allienne, J.F.; Boissier, J. Prevalence and risk factors associated with urogenital schistosomiasis among primary school pupils in Nigeria. Parasite Epidemiology and Control. Epidemiol. Control. 2022, 18, e00255. [Google Scholar] [CrossRef]
- Ayabina, D.V.; Clark, J.; Bayley, H.; Lamberton, P.H.L.; Toor, J.; Hollingsworth, T.D. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009083. [Google Scholar] [CrossRef]
- Dabo, A.; Agniwo, P.K.; Sidibe, B.; Diakite, A.; Diallo, M.; Niare, S.; Laurent Dembele, A.D. Impact de l’aménagement des points d’eau, une alternative efficace de lutte contre la schistosomose: Cas de la ville de Bamako. In Cahiers de l’observatoire Hommes-Milieux international Téssékéré; Cheikh Anta Diop University: Dakar, Senegal, 2022; p. 11. [Google Scholar]
- M’Bra, R.K.; Kone, B.; Yapi, Y.G.; Silué, K.D.; Sy, I.; Vienneau, D.; Soro, N.; Cissé, G.; Utzinger, J. Risk factors for schistosomiasis in an urban area in northern Côte d’Ivoire. Infect. Dis. Poverty 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Brooker, S.; Kabatereine, N.; Smith, J.; Mupfasoni, D.; Mwanje, M.; Ndayishimiye, O.; Lwambo, N.; Mbotha, D.; Mwandawiro, C.; Muchiri, E.; et al. An updated atlas of human helminth infections: The example of East Africa. Int J Heal. Geogr 2009, 8, 42. [Google Scholar] [CrossRef]
- Julius, E.; Siza Godfrey, M.; Kaatano, J.-Y.; Keeseon, S.; Eom, H.; Tai-Soon, Y. Prevalence of Schistosomes and Soil-Transmitted Helminths and Morbidity Associated with Schistosomiasis among Adult Population in Lake Victoria Basin, Tanzania. Korean J. Parasitol. 2015, 53, 5. [Google Scholar] [CrossRef]
- Nkosinathi, B.; Myra, T.; Siphosenkosi, C.; Zoulouun, L.; Sund, S.; Eyrun, F. Environnemental factors influencing the distribution and prevalence of Schistosoma haematobium in school attenders of ILembe and uThungulu Health Districts, KwaZulu-Natal Province, Soth Africa. South African J. Infect. Dis. 2017, 32, 132–137. [Google Scholar] [CrossRef]
- Okoli, C.G.; Iwuala, M.O.E. The prevalence, intensity and clinical signs of urinary schistosomiasis in Imo state, Nigeria. J. Helminthol. 2004, 78, 337. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.A.H.A.; Hong, S.T.; Babiker, A.T.E.B.; Hassan, R.M.A.E.; Sulaiman, M.A.Z.; Jeong, H.G.; Kong, W.H.; Lee, S.H.; Cho, H.I.; Nam, H.S.; et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasites Vectors 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ndeffo Mbah, M.L.; Poolman, E.M.; Drain, P.K.; Coffee, M.P.; van der Werf, M.J.; Galvani, A.P. HIV and Schistosoma haematobium prevalences correlate in sub-Saharan Africa. Trop. Med. Int. Heal. 2013, 18, 1174–1179. [Google Scholar] [CrossRef]
Figure 1.
Map of the district of Bamako showing the localization of the two study sites (schools in Taliko and Missabougou).
Figure 1.
Map of the district of Bamako showing the localization of the two study sites (schools in Taliko and Missabougou).
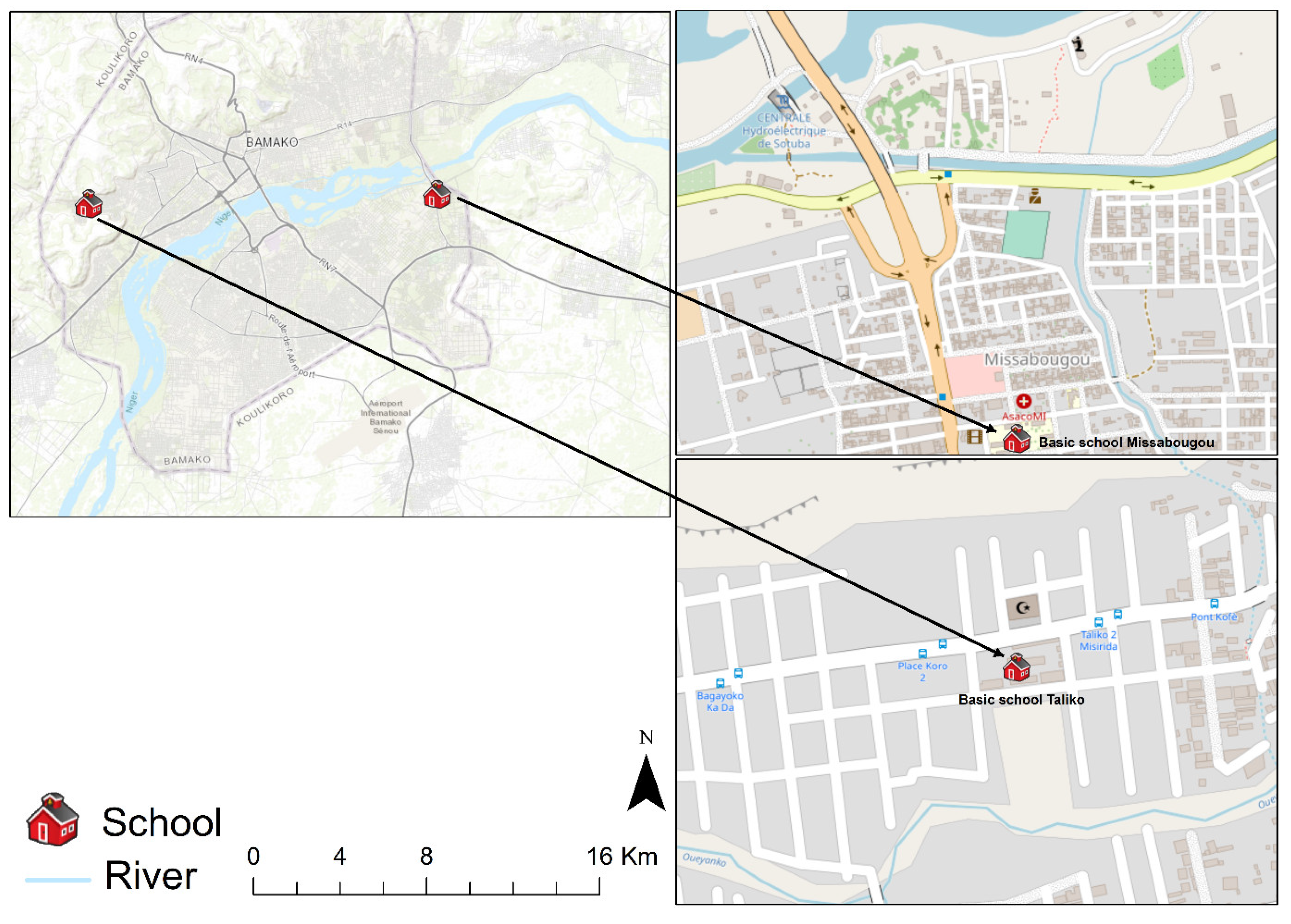
Figure 2.
Image of children’s bathing areas at the two study sites. A: Image of the clean–lined canal running alongside the Missabougou showing children bathing. B: Image showing the children fishing in Taliko.
Figure 2.
Image of children’s bathing areas at the two study sites. A: Image of the clean–lined canal running alongside the Missabougou showing children bathing. B: Image showing the children fishing in Taliko.

Table 1.
Prevalence of Schistosoma haematobium stratified by sites, gender, and age group among schoolchildren from the two study sites in Bamako, April 2023.
Table 1.
Prevalence of Schistosoma haematobium stratified by sites, gender, and age group among schoolchildren from the two study sites in Bamako, April 2023.
| Intensity | |||||||
|---|---|---|---|---|---|---|---|
| Site | No. of Children Examined | Prevalence n (%) | CI | Low n (%) | Heavy n (%) | p | |
| Missabougou | 378 | 11 (2.9) | [0–8.0] | <0.0001 | 11 (2.9) | 0 | <0.0001 |
| Taliko | 358 | 123 (34.4) | [29.1–39.6] | 114 (92.7) | 9 (7.3) | ||
| Gender | 0.11 | 0.003 | |||||
| Female | 401 | 66 (16.5) | [11.5– 21.4] | 66 (16.5) | 0 | ||
| Male | 335 | 68 (20.3) | [14.8– 25.7] | 59 (86.7) | 9 (13.2) | ||
| Age | <0.0001 | 0.001 | |||||
| [6–11[ | 207 | 21 (10.1) | [3.1–17.0] | 20 (9.7) | 1 (0.5) | ||
| [11–15[ | 529 | 113 (21.4) | [17.0–25.7] | 105 (92.9) | 8 (7.1) | ||
| Total | 736 | 134 (18.2) | [14.5–21.8] | 125 (17.0) | 9 (1.2) | ||
Table 2.
Prevalence and intensity of Schistosoma mansoni stratified by sites, gender, and age group among schoolchildren from the two study sites in Bamako, April 2023.
Table 2.
Prevalence and intensity of Schistosoma mansoni stratified by sites, gender, and age group among schoolchildren from the two study sites in Bamako, April 2023.
| Intensity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | No. of Children Examined | Prevalence n (%) | IC | p | Low | Moderate | Heavy | p |
| Missabougou | 345 | 15 (4.3) | [0–9.6] | 0.001 | 13 (86.8) | 1 (6.6) | 1 (6.6) | <0.0001 |
| Taliko | 323 | 39 (12.1) | [6.5–17.6] | 11 (3.4) | 4 (1.2) | 24 (7.4) | ||
| Gender | 0.45 | 0.80 | ||||||
| Female | 364 | 25 (6.9) | [1.6–12.1] | 6 (1.6) | 3 (0.8) | 16 (4.4) | ||
| Male | 304 | 29 (9.5) | [3.7–15.2] | 18 (5.9) | 2 (0.7) | 9 (3.0) | ||
| Age | 0.15 | 0.02 | ||||||
| [6–11[ | 187 | 9 (4.8) | [0–12.2] | 5 (2.7) | 0 | 4 (2.1) | ||
| [11–15[ | 481 | 45 (9.4) | [4.8–14] | 19 (4.0) | 5 (1.0) | 21 (4.4) | ||
| Total | 668 | 54 (8.1) | [4.2–11.9] | 24 (3.6) | 5 (0.7) | 25 (3.7) | ||
Table 3.
Multivariate analyses of human–water contact activities (HWCA) associated with Schistosoma spp. transmission among the sites, April 2023.
Table 3.
Multivariate analyses of human–water contact activities (HWCA) associated with Schistosoma spp. transmission among the sites, April 2023.
| Lafiabougou (Peri urban) | Missabougou (Urban) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Intensity | ||||||||||||||
| HWCA | Macro Hematuria | p | Micro Hematuria | p | Prevalence | p | Low | Heavy | p | Micro Hematuria | p | Prevalence | p | Low | p |
| Frequentation of the river | 0.83 | 0.0001 | 0.001 | 0.002 | 0.609 | 0.207 | 0.207 | ||||||||
| No | 2 (1.8) | 24 (21.8) | 24 (21.8) | 21 (87.5) | 3 (12.5) | 12 (5.8) | 4 (1.9) | 4 (100) | |||||||
| Yes | 6 (2.4) | 103 (41.5) | 99 (39.9) | 93 (93.9) | 6 (6.1) | 12 (7.1) | 7 (4.1) | 7 (100) | |||||||
| Domestic water source | 0.44 | 0.3 | 0.28 | 0.62 | 0.95 | 0.994 | 0.994 | ||||||||
| Rainwater | 0 | 1 (100) | 1 (100) | 1 (100) | 0 | 0 (0) | 0 (0) | 0 | |||||||
| Water source | 0 | 1 (100) | 1 (100) | 1 (100) | 0 | 0 (0) | 0 (0) | 0 | |||||||
| Tap water | 5 (1.7) | 105 (35.1) | 102 (34.1) | 94 (92.2) | 8 (7.8) | 22 (6.7) | 10 (3) | 10 (100) | |||||||
| Water bore hole | 3 (5.3) | 20 (35.1) | 19 (33.3) | 18 (94.7) | 1 (5.3) | 2 (4.5) | 1 (2.3) | 1 (100) | |||||||
| Well | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | ||||||
| Distance from school to river | 0.018 | 0.64 | 0.34 | 0.61 | 0.572 | 0.547 | 0.547 | ||||||||
| Far | 0 | 2 (33.3) | 3 (50) | 3 (100) | 0 | 8 (7.5) | 4 (3.7) | 4 (100) | |||||||
| Near | 8 (2.3) | 125 (35.5) | 120 (34.1) | 111 (92.5) | 9 (7.5) | 16 (5.9) | 7 (2.6) | 7 (100) | |||||||
| Urine in water | 0.78 | 0.01 | 0.001 | 0.006 | 0.75 | 0.851 | 0.851 | ||||||||
| No | 5 (2.7) | 54 (29.5) | 49 (28.8) | 46 (93.9) | 3 (6.1) | 14 (4.8) | 8 (2.8) | 8 (100) | |||||||
| Yes | 3 (1.7) | 73 (41.7) | 74 (42.3) | 68 (91.9) | 6 (8.1) | 10 (11.4) | 3 (3.4) | 3 (100) | |||||||
| Total | 8 | 127 | 123 | 114 | 9 | 24 | 11 | 11 | |||||||
Table 4.
Multivariate analyses of sociodemographic variables associated with Schistosoma haematobium infection by sites among schoolchildren in Taliko and Missabougou.
Table 4.
Multivariate analyses of sociodemographic variables associated with Schistosoma haematobium infection by sites among schoolchildren in Taliko and Missabougou.
| Taliko (Peri urban) | Missabougou (Urban) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Intensity | |||||||||||||||
| Sociodemographic varaiables | Micro Hematuria | p | Prevalence | p | Low | High | p | Total | Micro Hematuria | p | Prevalence | p | Low | High | p | Total |
| Age range | 0.001 | 0.188 | 0.36 | 0.8 | 0.06 | 0.06 | ||||||||||
| [6–11[ | 18 (25) | 20 (27.8) | 19 (95.0) | 1 (5.0) | 8 (5.9) | 1 (0.7) | 1 (100) | 0 | ||||||||
| [11–15[ | 109 (38.1) | 103 (36.0) | 95 (92.2) | 8 (7.8) | 16 (6.6) | 10 (4.1) | 10 (100) | 0 | ||||||||
| Gender | 0.168 | 0.001 | 0.16 | 0.004 | 0.004 | |||||||||||
| Female | 64 (30.9) | 0.035 | 65 (31.4) | 65 (100) | 0 | 9 (4.6) | 1 (0.5) | 1 (100) | 0 | |||||||
| Male | 63 (41.7) | 58 (38.4) | 49 (84.5) | 9 (15.5) | 15 (8.2) | 10 (5.4) | 10 (100) | 0 | ||||||||
| Parent Profession | 0.072 | 0.359 | 0.97 | 0.07 | 0.99 | |||||||||||
| Farmer | 2 (40) | 0.081 | 2 (40) | 2 (100) | 0 (0) | 4 (7.9) | 2 (3.7) | 2 (100) | 0 | |||||||
| Shopkeeper | 15 (33.2) | 13 (28.9) | 12 (92.2) | 1 (0.7) | 0 (0.0) | 0 (0) | 0 | 0 | ||||||||
| Civil servant | 5 (14.7) | 5 (14.7) | 5 (100) | 0 (0) | 3 (7.9) | 1 (2.6) | 1 (100) | 0 | ||||||||
| Gardener | 1 (100) | 1 (100) | 1 (100) | 0 (0) | ||||||||||||
| Worker | 104 (38.2) | 102 (37.5) | 94 (92.2) | 8 (7.8) | 17 (6) | 8 (2.8) | 8 (100) | 0 | ||||||||
| Fisherman | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0) | 0 | 0 | ||||||||
| Total | 127 | 123 (34.4) | 114 | 9 | 358 | 24 | 11 (2.9) | 11 | 0 | 378 | ||||||
Table 5.
Multivariate analyses of clinical signs associated with Schistosoma haematobium infection among sites, April 2023.
Table 5.
Multivariate analyses of clinical signs associated with Schistosoma haematobium infection among sites, April 2023.
| Taliko (Peri Urban) | Missabougou (Urban) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Intensity | ||||||||||||||
| Clinical Signs | Macro_Hematuria | p | Micro Hematuria | p | Prevalence | p | Low | High | p | Micro Hematuria | p | Prevalence | p | Low | p |
| Bladder pain | 0.72 | 0.0001 | 0.0001 | 0.0001 | 0.18 | 0.78 | 0.78 | ||||||||
| No | 5 (2.3) | 62 (28.1) | 54 (24.4) | 51 (94.4) | 3 (4.6) | 21 (7.3) | 8 (2.8) | 8 (100) | |||||||
| Yes | 3 (2.2) | 65 (47.4) | 69 (50.4) | 63 (91.3) | 6 (8.7) | 3 (3.3) | 3 (3.3) | 3 (100) | |||||||
| Pollakiuria | 0.15 | 0.19 | 0.36 | 0.905 | 0.4 | 0.06 | 0.06 | ||||||||
| No | 6 (2.2) | 94 (34.1) | 93 (33.7) | 86 (92.5) | 7 (7.5) | 17 (6.3) | 5 (1.8) | 5 (100) | |||||||
| Yes | 2 (2.4) | 33 (40.2) | 30 (36.6) | 28 (93.3) | 2 (6.7) | 7 (7.5) | 6 (6.5) | 6 (100) | |||||||
| Dysuria | 0.18 | 0.006 | 0.003 | 0.007 | 0.4 | 0.25 | 0.25 | ||||||||
| No | 4 (1.5) | 85 (31.6) | 81 (30.1) | 75 (92.6) | 6 (7.4) | 17 (5.8) | 7 (2.4) | 7 (100) | |||||||
| Yes | 4 (4.5) | 42 (47.2) | 42 (47.2) | 39 (92.9) | 3 (7.1) | 7 (8.3) | 4 (4.8) | 4 (100) | |||||||
| Total | 8 | 127 | 123 | 114 | 9 | 24 | 11 | 11 | |||||||
© 2024 Copyright by Authors. Licensed as an open access article using a CC BY 4.0 license.

